Abstract
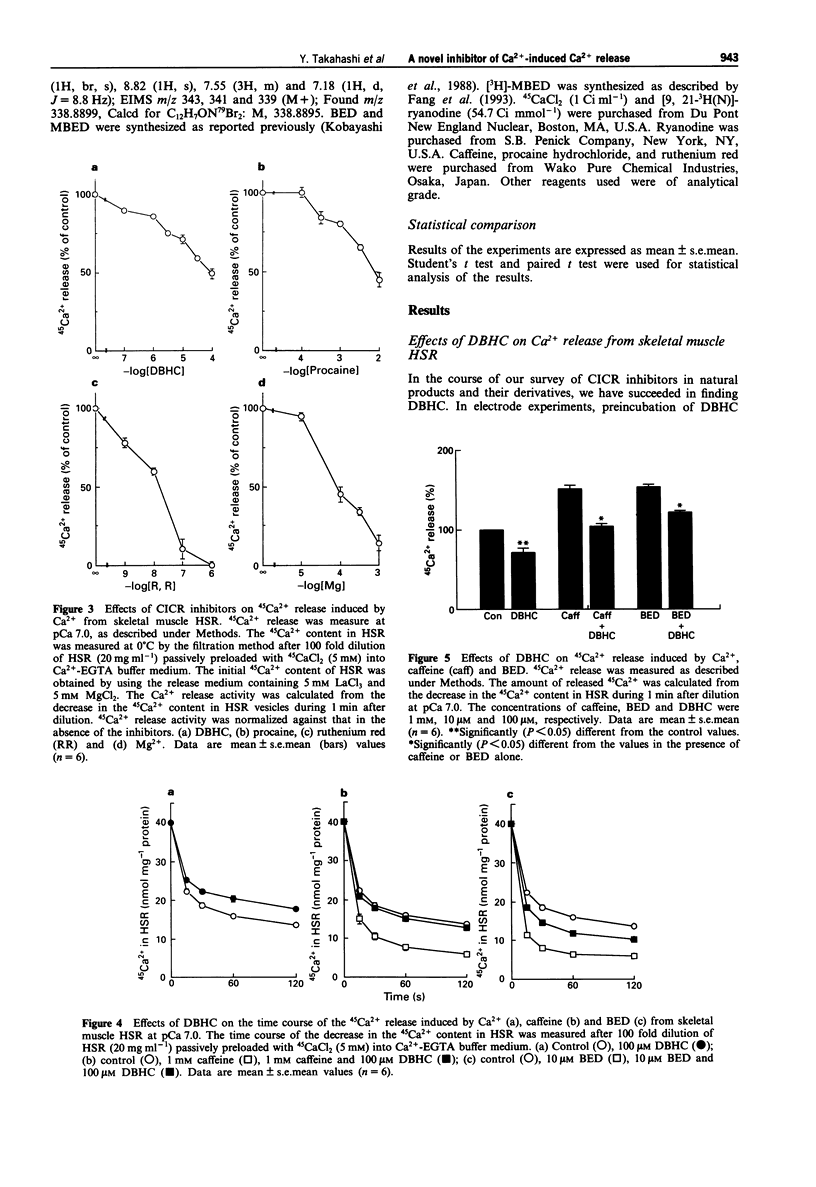
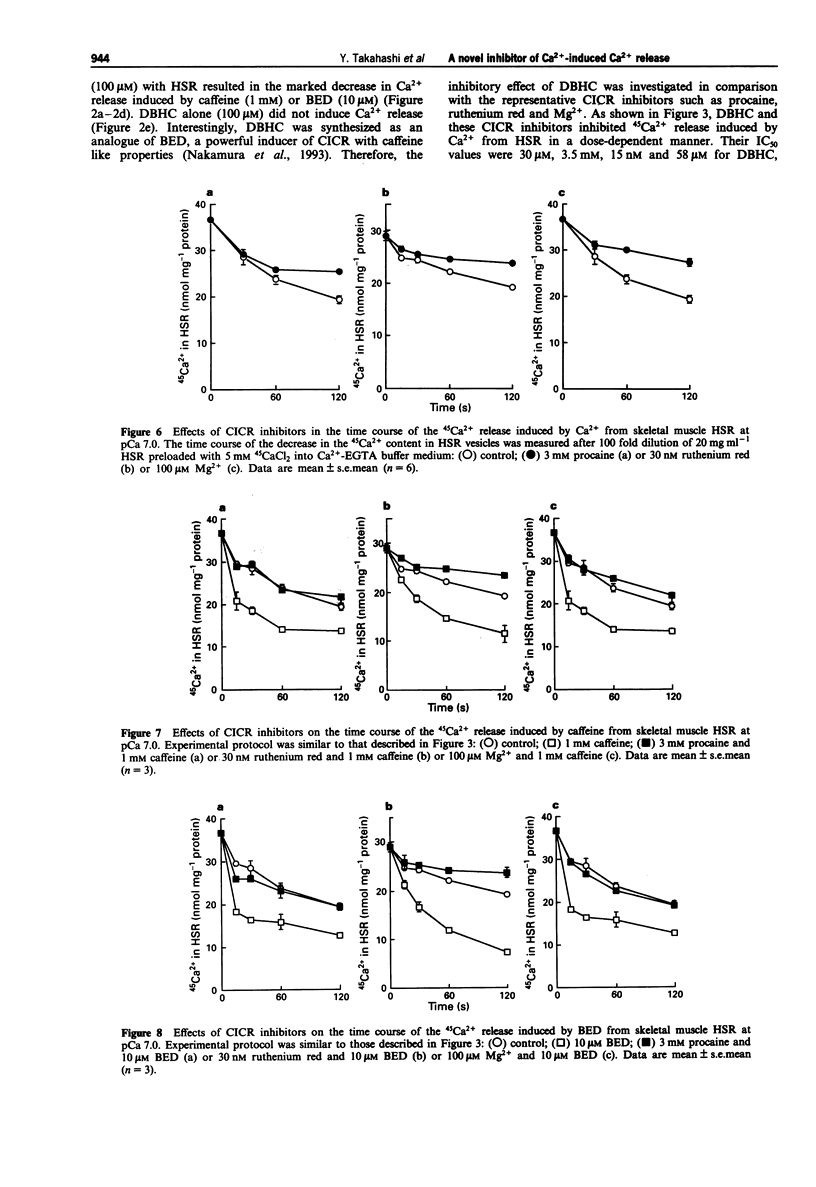
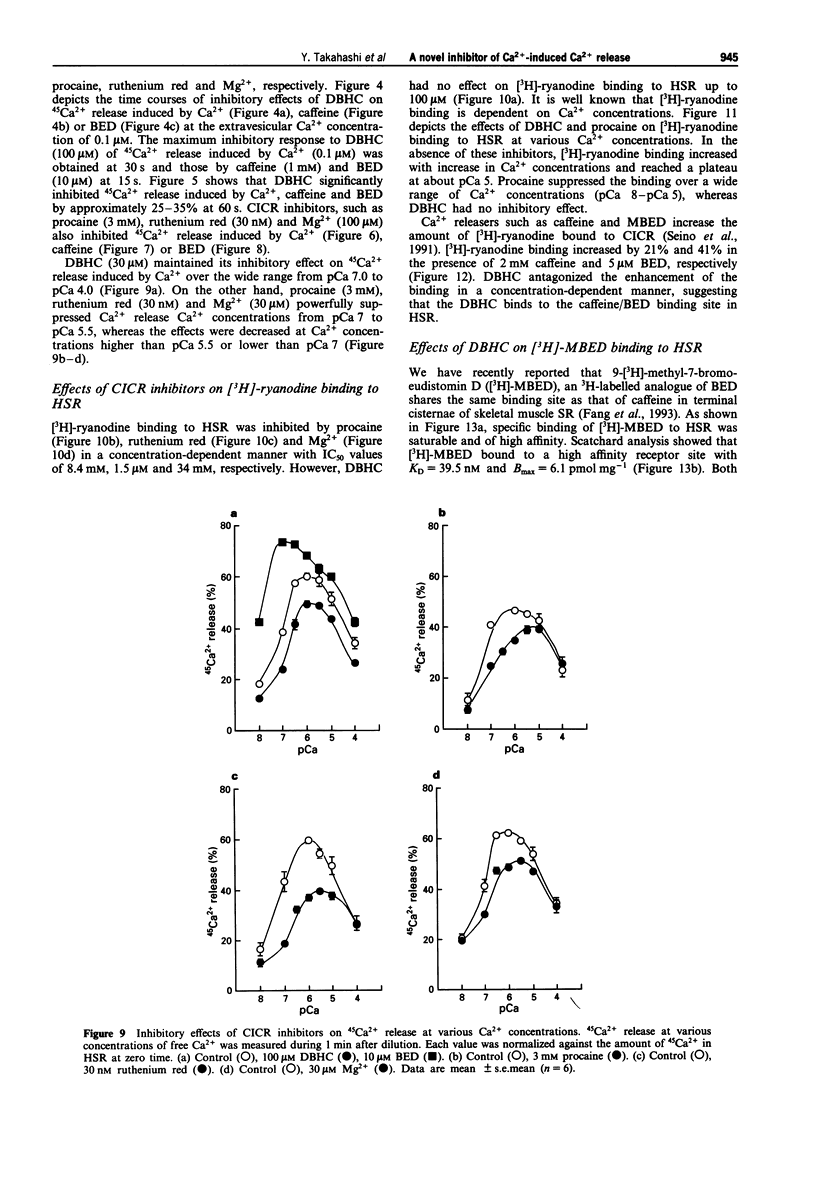
1. 4,6-Dibromo-3-hydroxycarbazole (DBHC) was synthesized as an analogue of bromoeudistomin D (BED), a powerful Ca2+ releaser, and its pharmacological properties were examined. 2. In Ca2+ electrode experiments, DBHC (100 microM) markedly inhibited Ca2+ release from the heavy fraction of sarcoplasmic reticulum (HSR) induced by caffeine (1 mM) and BED (10 microM). 3. DBHC (0.1 to 100 microM) inhibited 45Ca2+ release induced by Ca2+ from HSR in a concentration-dependent manner. 4. DBHC (100 microM) abolished 45Ca2+ release induced by caffeine (1 mM) and BED (10 microM) in HSR. 5. Inhibitory effects of calcium-induced calcium release (CICR) blockers such as procaine, ruthenium red and Mg2+ on 45Ca2+ release were clearly observed at Ca2+ concentrations from pCa 7 to pCa 5.5, and were decreased at Ca2+ concentrations higher than pCa 5.5 or lower than pCa 7. However, DBHC decreased Ca2+ release induced by Ca2+ over the wide range of extravesicular Ca2+ concentrations. 6. [3H]-ryanodine binding to HSR was suppressed by ruthenium red, Mg2+ and procaine, but was not affected by DBHC up to 100 microM. 7. [3H]-ryanodine binding to HSR was enhanced by caffeine and BED. DBHC antagonized the enhancement in a concentration-dependent manner. 8. 9-[3H]-Methyl-7-bromo-eudistomin D, an 3H-labelled analogue of BED, specifically bound to HSR. Both DBHC and caffeine increased the KD value without affecting the Bmax value, indicating a competitive mode of inhibition. 9. These results suggest that DBHC binds to the caffeine binding site to block Ca2+ release from HSR.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF
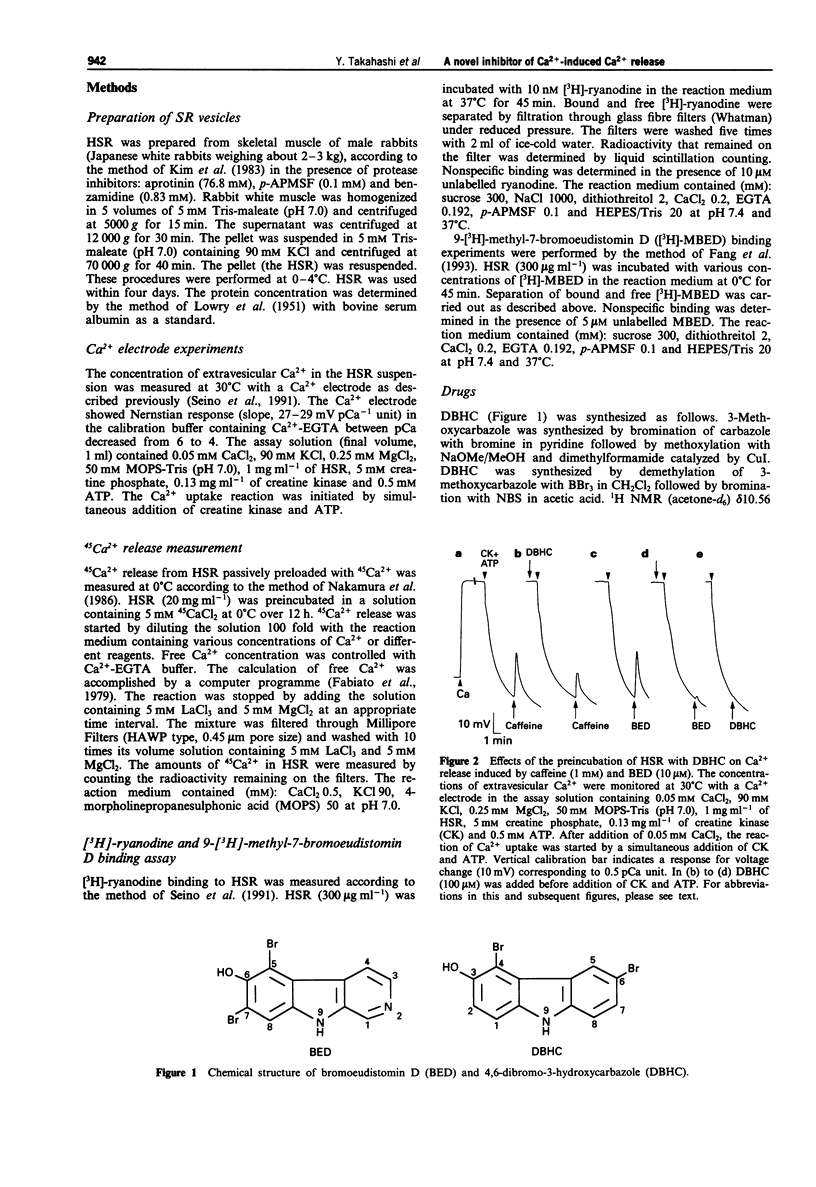


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Corbalan-Garcia S., Teruel J. A., Gomez-Fernandez J. C. Characterization of ruthenium red-binding sites of the Ca(2+)-ATPase from sarcoplasmic reticulum and their interaction with Ca(2+)-binding sites. Biochem J. 1992 Nov 1;287(Pt 3):767–774. doi: 10.1042/bj2870767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S. Excitation-contraction coupling and the mechanism of muscle contraction. Annu Rev Physiol. 1991;53:1–16. doi: 10.1146/annurev.ph.53.030191.000245. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fang Y. I., Adachi M., Kobayashi J., Ohizumi Y. High affinity binding of 9-[3H]methyl-7-bromoeudistomin D to the caffeine-binding site of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1993 Sep 5;268(25):18622–18625. [PubMed] [Google Scholar]
- Fleischer S., Ogunbunmi E. M., Dixon M. C., Fleer E. A. Localization of Ca2+ release channels with ryanodine in junctional terminal cisternae of sarcoplasmic reticulum of fast skeletal muscle. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7256–7259. doi: 10.1073/pnas.82.21.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Calcium uptake and force development by skinned muscle fibres in EGTA buffered solutions. J Physiol. 1972 May;223(1):1–19. doi: 10.1113/jphysiol.1972.sp009830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa T., Smith J. S., Coronado R., Campbell K. P. Purified ryanodine receptor from skeletal muscle sarcoplasmic reticulum is the Ca2+-permeable pore of the calcium release channel. J Biol Chem. 1987 Dec 5;262(34):16636–16643. [PubMed] [Google Scholar]
- Inui M., Saito A., Fleischer S. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J Biol Chem. 1987 Feb 5;262(4):1740–1747. [PubMed] [Google Scholar]
- Kim D. H., Ohnishi S. T., Ikemoto N. Kinetic studies of calcium release from sarcoplasmic reticulum in vitro. J Biol Chem. 1983 Aug 25;258(16):9662–9668. [PubMed] [Google Scholar]
- Kirino Y., Shimizu H. Ca2+-induced Ca2+ release from fragmented sarcoplasmic reticulum: a comparison with skinned muscle fiber studies. J Biochem. 1982 Oct;92(4):1287–1296. doi: 10.1093/oxfordjournals.jbchem.a134047. [DOI] [PubMed] [Google Scholar]
- Kobayashi J., Ishibashi M., Nagai U., Ohizumi Y. 9-Methyl-7-bromoeudistomin D, a potent inducer of calcium release from sarcoplasmic reticulum of skeletal muscle. Experientia. 1989 Aug 15;45(8):782–783. doi: 10.1007/BF01974589. [DOI] [PubMed] [Google Scholar]
- Kobayashi J., Taniguchi M., Hino T., Ohizumi Y. Eudistomin derivatives, novel phosphodiesterase inhibitors: synthesis and relative activity. J Pharm Pharmacol. 1988 Jan;40(1):62–63. doi: 10.1111/j.2042-7158.1988.tb05154.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- McPherson P. S., Campbell K. P. The ryanodine receptor/Ca2+ release channel. J Biol Chem. 1993 Jul 5;268(19):13765–13768. [PubMed] [Google Scholar]
- Michalak M., Dupraz P., Shoshan-Barmatz V. Ryanodine binding to sarcoplasmic reticulum membrane; comparison between cardiac and skeletal muscle. Biochim Biophys Acta. 1988 Apr 22;939(3):587–594. doi: 10.1016/0005-2736(88)90106-x. [DOI] [PubMed] [Google Scholar]
- Nagasaki K., Kasai M. Channel selectivity and gating specificity of calcium-induced calcium release channel in isolated sarcoplasmic reticulum. J Biochem. 1984 Dec;96(6):1769–1775. doi: 10.1093/oxfordjournals.jbchem.a135009. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Kobayashi J., Gilmore J., Mascal M., Rinehart K. L., Jr, Nakamura H., Ohizumi Y. Bromo-eudistomin D, a novel inducer of calcium release from fragmented sarcoplasmic reticulum that causes contractions of skinned muscle fibers. J Biol Chem. 1986 Mar 25;261(9):4139–4142. [PubMed] [Google Scholar]
- Pessah I. N., Francini A. O., Scales D. J., Waterhouse A. L., Casida J. E. Calcium-ryanodine receptor complex. Solubilization and partial characterization from skeletal muscle junctional sarcoplasmic reticulum vesicles. J Biol Chem. 1986 Jul 5;261(19):8643–8648. [PubMed] [Google Scholar]
- Pessah I. N., Stambuk R. A., Casida J. E. Ca2+-activated ryanodine binding: mechanisms of sensitivity and intensity modulation by Mg2+, caffeine, and adenine nucleotides. Mol Pharmacol. 1987 Mar;31(3):232–238. [PubMed] [Google Scholar]
- Rousseau E., Ladine J., Liu Q. Y., Meissner G. Activation of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum by caffeine and related compounds. Arch Biochem Biophys. 1988 Nov 15;267(1):75–86. doi: 10.1016/0003-9861(88)90010-0. [DOI] [PubMed] [Google Scholar]
- Seino A., Kobayashi M., Kobayashi J., Fang Y. I., Ishibashi M., Nakamura H., Momose K., Ohizumi Y. 9-methyl-7-bromoeudistomin D, a powerful radio-labelable Ca++ releaser having caffeine-like properties, acts on Ca(++)-induced Ca++ release channels of sarcoplasmic reticulum. J Pharmacol Exp Ther. 1991 Mar;256(3):861–867. [PubMed] [Google Scholar]
- Sorrentino V., Volpe P. Ryanodine receptors: how many, where and why? Trends Pharmacol Sci. 1993 Mar;14(3):98–103. doi: 10.1016/0165-6147(93)90072-r. [DOI] [PubMed] [Google Scholar]
- Wagenknecht T., Grassucci R., Frank J., Saito A., Inui M., Fleischer S. Three-dimensional architecture of the calcium channel/foot structure of sarcoplasmic reticulum. Nature. 1989 Mar 9;338(6211):167–170. doi: 10.1038/338167a0. [DOI] [PubMed] [Google Scholar]


