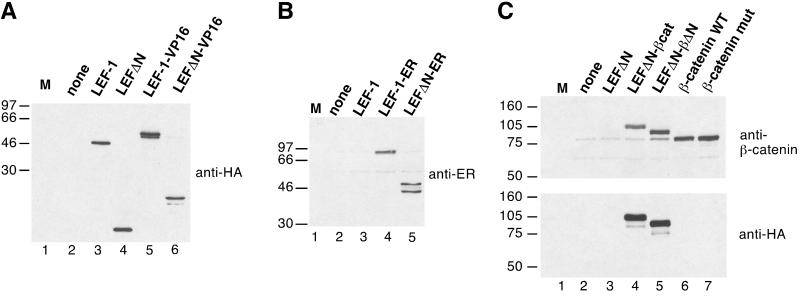
Figure 2.
(A) Expression of chimeric LEF-1 constructs. Human 293 cells were transfected with empty expression vector (lane 2) or expression vectors for LEF-1, LEFΔN, LEF-1-VP16, or LEFΔN-VP16. Whole cell lysates were prepared and resolved by electrophoresis in an SDS/10% polyacrylamide gel. Proteins were transferred onto nitrocellulose and probed with a monoclonal antibody recognizing the HA tag. (B) Cellular lysates from 293 cells transfected with empty expression vector (lane 2) or expression vectors for LEF-1, LEF-1-ER, or LEFΔN-ER were resolved by electrophoresis in an SDS/10% polyacrylamide gel and transferred onto nitrocellulose. The blot was probed with a polyclonal antibody directed against the hormone-binding domain of the human ER. (C) Cellular lysates from Neuro2a cells transfected with empty expression vector (lane 2) or expression vectors for LEFΔN, LEFΔNβ-cat, LEFΔN-βΔN, or wild-type (WT) and mutant β-catenin, respectively, as indicated were resolved by electrophoresis in SDS/7.5% polyacrylamide gels and transferred onto nitrocellulose. The upper blot was probed with a monoclonal antibody directed against β-catenin. The faint band visible in lanes 2–5 that migrates at the same position as the transfected β-catenin (lanes 6 and 7) represents endogenous protein of Neuro2a cells. The lower blot was probed with a monoclonal antibody against the HA tag. M lanes contain molecular size markers. The mass of the marker proteins in kDa is shown on the left.