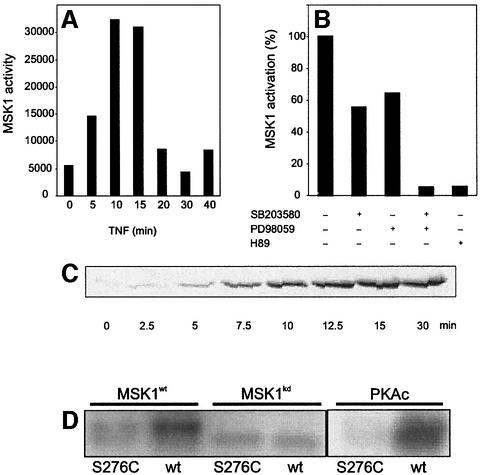
Fig. 4. MSK1 is activated by TNF in vivo and phosphorylates Ser276 of p65 in vitro. (A) L929sA cells were starved for 48 h in serum-free medium and stimulated with 2000 IU/ml TNF. Cells were lysed and endogenous MSK1 was isolated by immunoprecipitation. The activity of MSK1 was assessed by an in vitro kinase assay. (B) After 2 days of serum starvation, L929sA cells were incubated for 4 h in serum-free medium supplemented with 10 µM SB203580, 10 µM PD98059 or a combination. Cells were treated with 2000 IU/ml TNF for 15 min in the presence or absence of these inhibitors. After cell lysis, MSK1 was immunoprecipitated and assayed for its ability to phosphorylate CREBtide. Where indicated, H89 was included in the in vitro reaction. (C) L929sA cells were treated with 2000 IU/ml TNF. The presence of p65 in the nuclear extracts was revealed by western blotting. (D) MSK1 was isolated from HEK293 cells overexpressing either wt MSK1 or a kinase-dead mutant, together with the upstream activators p38 and MKK6. Immunoprecipitates were used in an in vitro kinase reaction with either wt GSTp6512–317 or the corresponding Ser276 mutant (S276C) for 20 min at 30°C, followed by SDS–PAGE. Similar experiments were performed with 15 ng of purified PKAc.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.