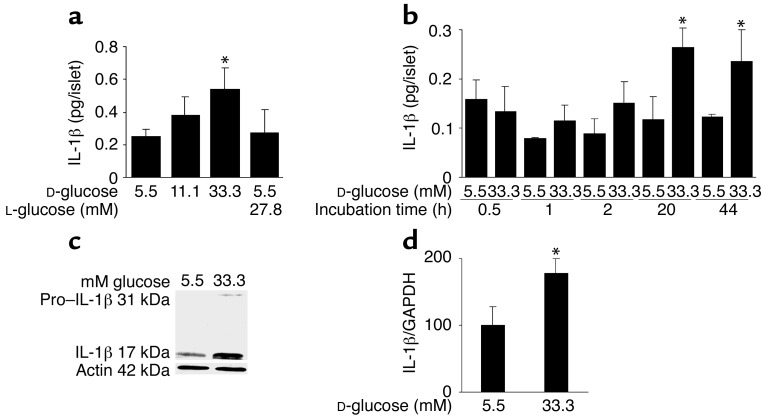
Figure 1.
Glucose induces IL-1β expression and release in human islets. (a) Secretion of IL-1β from human islets cultured on extracellular matrix–coated dishes for 4 days in 5.5, 11.1, or 33.3 mM D-glucose or in 5.5 mM D-glucose plus 27.8 mM L-glucose. Each bar represents the mean ± SEM of eight experiments from eight separate donors. *P < 0.01 compared with islets cultured in 5.5 mM glucose alone. (b) Secretion of IL-1β from human islets during 44 hours of culture in suspension with 5.5 or 33.3 mM D-glucose. Data were collected from four tubes per treatment in two separate experiments from two donors. Data are represented as mean ± SEM. *P < 0.01 compared with islets cultured in 5.5 mM glucose. (c) Immunoblotting of pro–IL-1β, IL-1β, and actin. Human islets cultured in suspension at 5.5 or 33.3 mM glucose were analyzed after 44 hours of incubation. One experiment of eleven (from eleven donors) is shown. In seven experiments, glucose induced IL-1β. In three experiments, IL-1β remained unchanged, and in one it was decreased. (d) RT-PCR detection and quantification of IL-1β mRNA expression. Total RNA was isolated from human islets cultured for 44 hours in medium containing 5.5 or 33.3 mM glucose. In the LightCycler quantitative PCR system, the level of IL-1β expression was normalized against GAPDH and the results were expressed as mRNA levels relative to control incubations at 5.5 mM. Results are presented as mean ± SEM for six independent experiments from six donors. *P < 0.05 relative to islets cultured in 5.5 mM glucose.