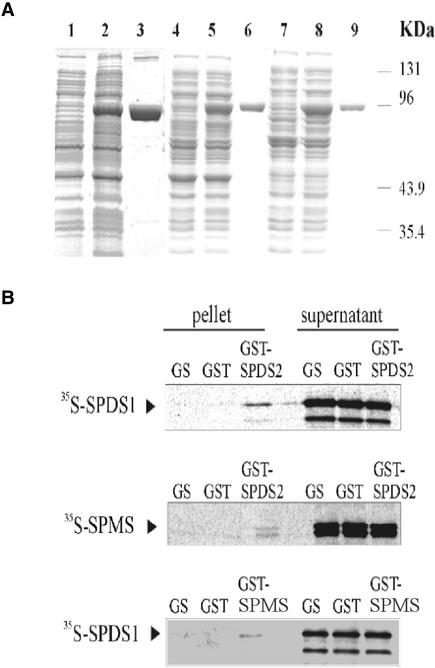
Figure 4.
Interactions of SPDS and SPMS Proteins in Vitro.
(A) The SPDS and SPMS proteins were fused to N-terminal GST tags using pGEX vectors and purified from E. coli using glutathione-Sepharose affinity chromatography. Quality tests of total protein extracts from E. coli before (lanes 1, 4, and 7) and after (lanes 2, 5, and 8) induction of GST-SPDS/SPMS expression with isopropylthiogalactoside, and that of purified GST-SPDS/SPMS proteins (lanes 3, 6, and 9), were performed by SDS-PAGE. Lanes 1 to 3, GST-SPDS1; lanes 4 to 6, GST-SPMS; and lanes 7 to 9, GST-SPDS2.
(B) SDPS1 and SPMS labeled with 35S-Met were subjected to pulldown assay with GST-SPDS2. Beads carrying immobilized GST-SPDS2 and control GST proteins, as well as the control glutathione-Sepharose matrix (GS), were incubated with equal amounts of labeled SPDS proteins. The eluted matrix-bound proteins and aliquots from the supernatants were separated by SDS-PAGE and detected by autoradiography. A similar pulldown assay was performed using 35S-SPDS1 and GST-SPMS.