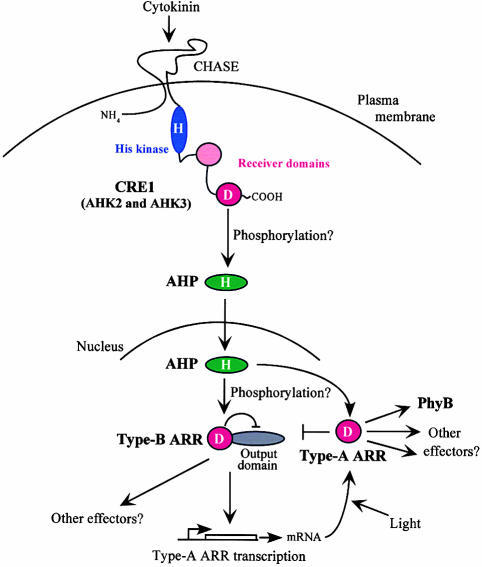
Figure 4.
Model for Phosphorelay Signal Transduction in Cytokinin Signaling.
Cytokinin binds to CRE1, and possibly other His kinase–like proteins such as AHK2 and AHK3, within the CHASE domain, which is flanked by predicted transmembrane domains. CRE1 is likely to be located in the plasma membrane and, by analogy to other histidine kinases, to act as a dimer (not shown in model). The binding of cytokinin activates the transmitter domain (blue), which autophosphorylates on a His (H). The phosphate then is transferred to an Asp residue (D) within the fused receiver domain (red). A second, degenerate receiver domain (pink) also is present. The phosphate then is likely to be transferred to an AHP protein, which translocates to the nucleus, where it activates type-B ARRs. The activated type-B ARRs increase the transcription of the type-A ARRs, which feed back to inhibit their own transcription (⊥). Light appears to increase ARR4 protein levels, as indicated by the arrow. The receiver domain of type-B ARRs inhibits the activity of the output domain (gray). The output of this signaling pathway is mostly unknown, although one likely target is PhyB. See text for additional details.