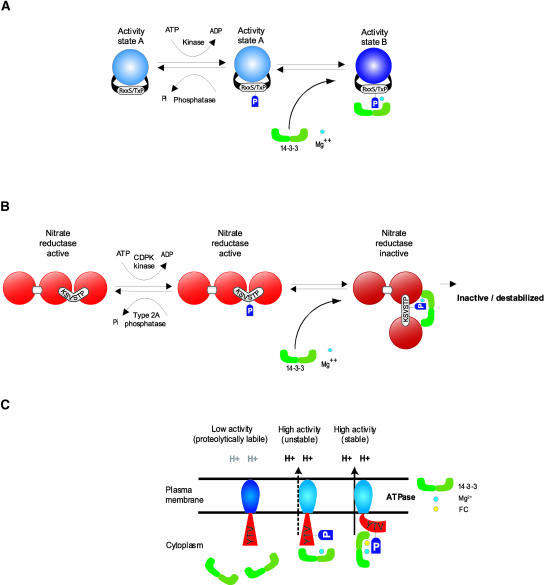
Figure 1.
Consummated Signal Transduction.
(A) The basic paradigm for 14-3-3 regulation is a two-step process whereby the target protein is phosphorylated by a kinase but remains in its initial activity state until 14-3-3s bind in the presence of divalent cation to complete the transition to a new activity state.
(B) NR regulation by 14-3-3s is an extension of the basic paradigm. In its unphosphorylated state, NR is active. Phosphorylation by CDPK does not inactivate NR, but phosphorylation does tag the enzyme for 14-3-3 binding, which completes the signal-induced transition toward inactivation. NR that is phosphorylated and bound by 14-3-3s may be inactivated directly in a reversible fashion or may be destabilized and subjected to proteolysis.
(C) Aspects of the paradigm model apply to the apparently specialized case of 14-3-3 regulation of H+-ATPase, in which binding in the presence of FC locks the enzyme into a highly active state. In the absence of FC, 14-3-3s bind to increase the activity of H+-ATPase in a regulatable manner.