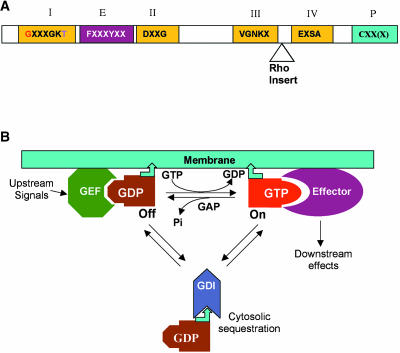
Figure 1.
Conserved Structure and Regulation of Small GTPases.
(A) Conserved structure of small GTPase. All small GTPases contain four conserved domains for guanine nucleotide binding and GTPase activities (I through IV) and an effector domain (E). Residues in red have been used for the generation of CA mutants, and those in purple for DN mutants (see Figure 2). The Rho insert (10 to 12 amino acids) is only found in Rho GTPases. Rab, Ras, and Rho GTPases also contain a C-terminal motif for prenylation (P). The motif can be CAAL (C, cysteine; A, aliphatic amino acid; L, leucine) for geranylgeranylation by geranylgeranyltransferase I (GGTase I), CC/CXC for geranylgeranylation by GGTase II, or CAAX (X indicates any amino acid except for leucine and phenyalanine) for farnesylation. Arf is myristoylated at the N terminus, but Ran has no known modification.
(B) A general scheme for the regulation and action of small GTPases. GAP, GTPase-activating protein; GDI, guanine nucleotide dissociation inhibitor; GEF, guanine nucleotide exchange factor. Bent arrowhead indicates a lipid moiety that becomes attached to membranes.