Figure 4. Implantation of age- and risk-matched human TEBV in non-human primates.
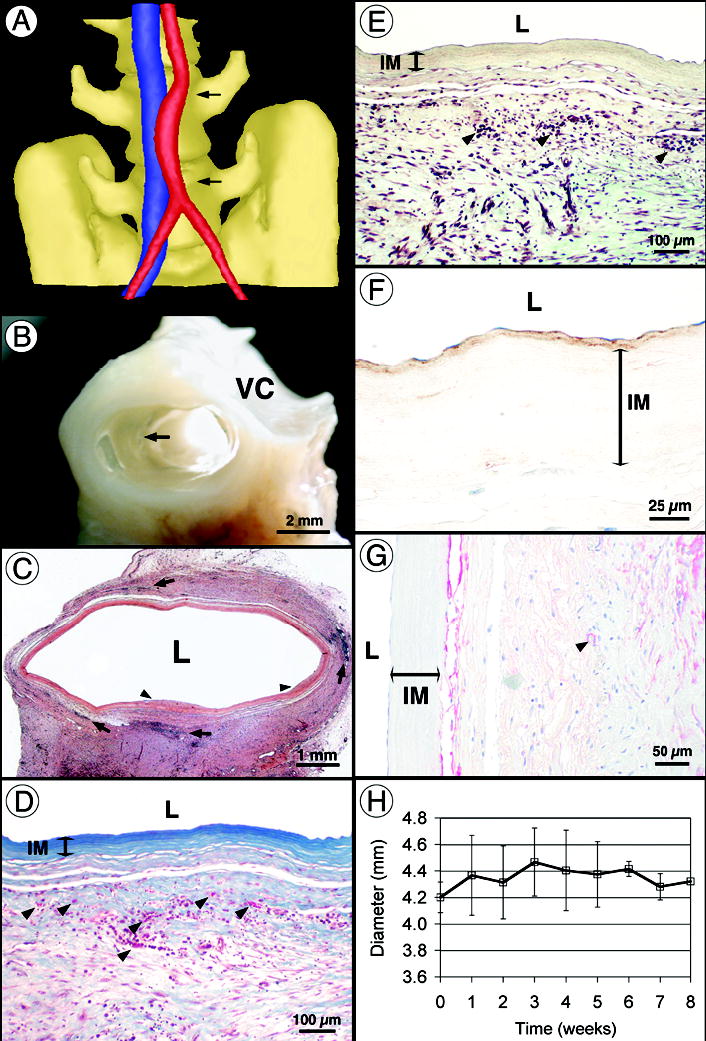
(a) Computed tomography angiogram after 8 weeks of implantation showing a patent TEBV (red) and the adjacent vena cava (blue). Note that the initial diameter mismatch is still visible in the middle of the graft and that the curved shape is typical of an interpositional graft. (b) Perfusion-fixed TEBV at 8 weeks shows complete tissue integration and a smooth lumen with no signs of thrombosis, stenosis or mechanical failure. Arrow indicates suture line. VC: vena cava. (c) Histology (H&E) of the graft at 8 weeks shows multiple leukocyte infiltrations (arrows) and rare sites of limited luminal growth (arrow heads). (d–e) At higher magnification, Verhoff-Masson and Movat stainings show an intact and still acellular IM. Note that proteoglycans are no longer present in the TEBV. Vasa vasorum formation was noted (arrows in D). The significant immune response observed (arrows in e) may be responsible for 18 incomplete tissue integration. (f) Immunolabeling for von Willebrand factor reveals the confluent endothelium and subendothelial matrix (nuclei counterstained in blue). (g) Immunolabeling for SMC-specific α-actin stains a cell population at the IM/adventitia interface (blue counterstain). Arrow indicates small blood vessel. (h) TEBV internal diameter was measured at the center of the graft by weekly ultrasound-Doppler examination of all animals. After an increase of about 5% associated with initial pressurization, the diameter remained constant for the duration of the study.
