Fig. 1.
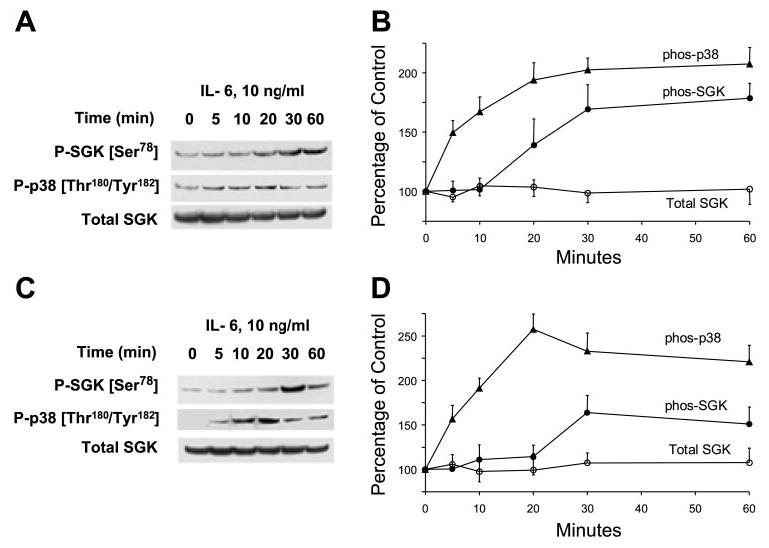
Interleukin-6 (IL-6) stimulates p38 MAP kinase activation and glucocorticoid-stimulated kinase (SGK) phosphorylation at Ser78 site. A and C: representative immunoblots are shown with quantitative data after densitometric analysis from 3 separate experiments (B and D). Malignant human cholangiocarcinoma cell lines KMCH (A and B) and Mz-ChA-1 (C and D) were serum starved for 12 h before incubation with 10 ng/ml IL-6. At the indicated times, cell lysates were prepared and analyzed by immunoblotting with the use of a Thr180/Tyr182 phospho-specific p38 MAPK antibody (P-p38). The blot was stripped and reprobed with antibodies specific for phospho-Ser78 and phosphorylation site-independent SGK (Total SGK). In both KMCH and Mz-ChA-1 cells, p38 MAPK phosphorylation occurred within 5 min of stimulation with IL-6 and preceded increases in SGK phosphorylation, which were first detected 20–30 min after stimulation.
