Fig. 3.
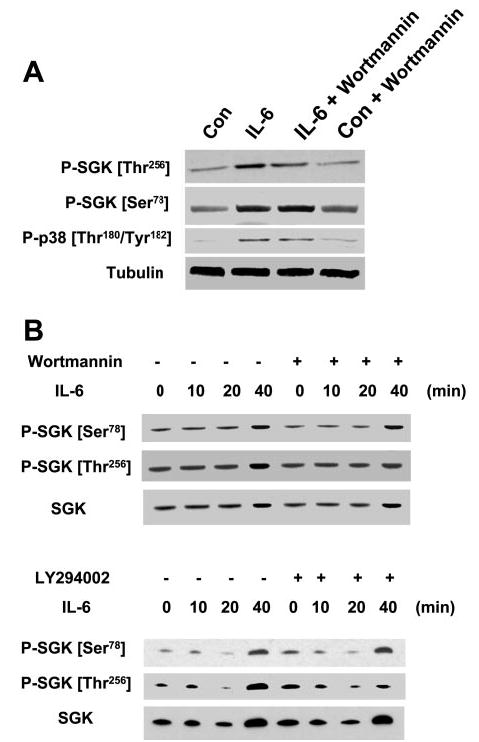
Inhibition of phosphatidylinositol 3-kinase (PI3-kinase) blocks IL-6-induced phosphorylation of SGK at the Thr256 site but not at the Ser78 site. A: KMCH cells were incubated with or without 10 ng/ml IL-6 in the presence or absence of the PI3-kinase inhibitor wortmannin (1 μM). After 40 min, cells were lysed and immunoblot analysis performed using phosphorylation site-specific antibodies to SGK Thr256, SGK Ser78, p38 MAPK Thr180/Tyr182, and α-tubulin. Wortmannin blocked SGK phosphorylation at Thr256, but not at Ser78. Identical results were obtained with LY-294002 (100 μM) and lower concentrations of wortmannin (100–500 nM) (not shown). B: KMCH cells were incubated with IL-6 (10 ng/ml) in the presence or absence of 500 nM wortmannin or 50 μM LY-294002. At the indicated times, nuclear extracts were obtained as described in materials and methods. Immunoblot analysis was performed using SGK Ser78 and Thr256 phosphorylation site-specific and total SGK antibodies. Nuclear expression of total SGK and SGK Ser78, indicating phosphorylation site-specific activation and translocation of SGK, occurred despite inhibition of PI3-kinase.
