Fig. 5.
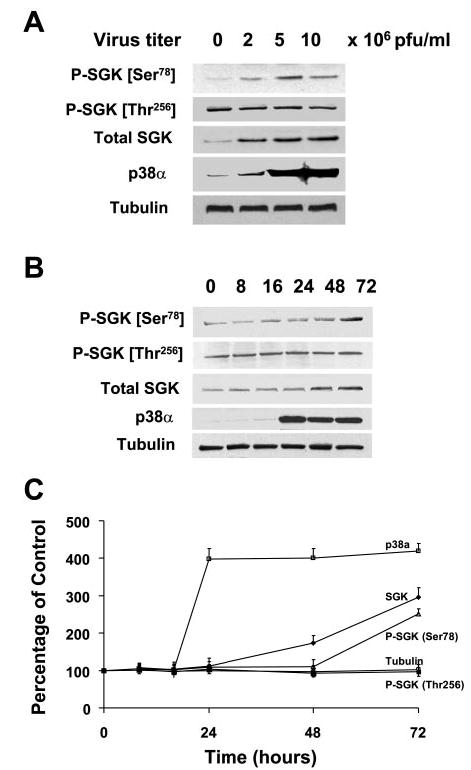
Overexpression of p38α stimulates SGK phosphorylation at Ser78 in the absence of IL-6. A: KMCH cells were infected with 0, 2, 5, and 10 × 106 plaque-forming units (PFU)/ml of adenovirus encoding p38α MAPK cDNA. Whole cell lysates were prepared 48 h after infection and assessed by immunoblot analysis with a Ser78 phospho-specific SGK antibody. The blots were stripped and reprobed with phospho-specific antibodies against Thr256 SGK or phosphorylation state independent antibodies to SGK (Total SGK), p38 MAPK, and α-tubulin (loading control). SGK phosphorylation at Ser78 but not at Thr256 increased proportionally to p38 MAPK expression. B: representative blot. C: means ± SD from 3 separate experiments. In B and C, cell lysates were obtained at various time points after infection as indicated above the lanes. Immunoblot analysis was performed using Ser78 or Thr256 phospho-specific SGK antibodies. The blot was stripped and reprobed with an antibody against total SGK, p38α MAPK, and α-tubulin. Increased p38α expression was seen within 24 h, whereas SGK expression was evident only 36 h after infection. An increase in Ser78 but not Thr256 phosphorylation was observed after 48 h.
