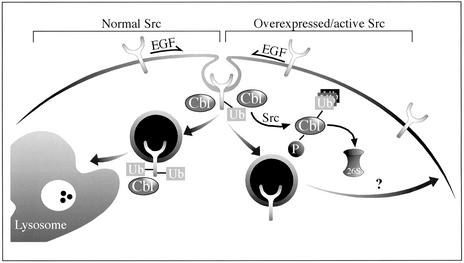
Figure 5.
Proposed mode of interactions between c-Src and c-Cbl and their effect on EGFR trafficking. Normally, EGF promotes receptor phosphorylation, followed by recruitment of c-Cbl, receptor ubiquitylation, and sorting of EGFR to lysosomal degradation. In the presence of an active/overexpressed c-Src, both phosphorylation and ubiquitylation of c-Cbl are enhanced, and the protein is subsequently degraded by the 26S proteasome. Because of Src-induced elimination of c-Cbl, sorting of EGFR to lysosomal degradation is reduced, and the receptor is diverted to the recycling pathway. This regulatory loop may explain the association between Src activation and EGFR over-expression in tumor cells.