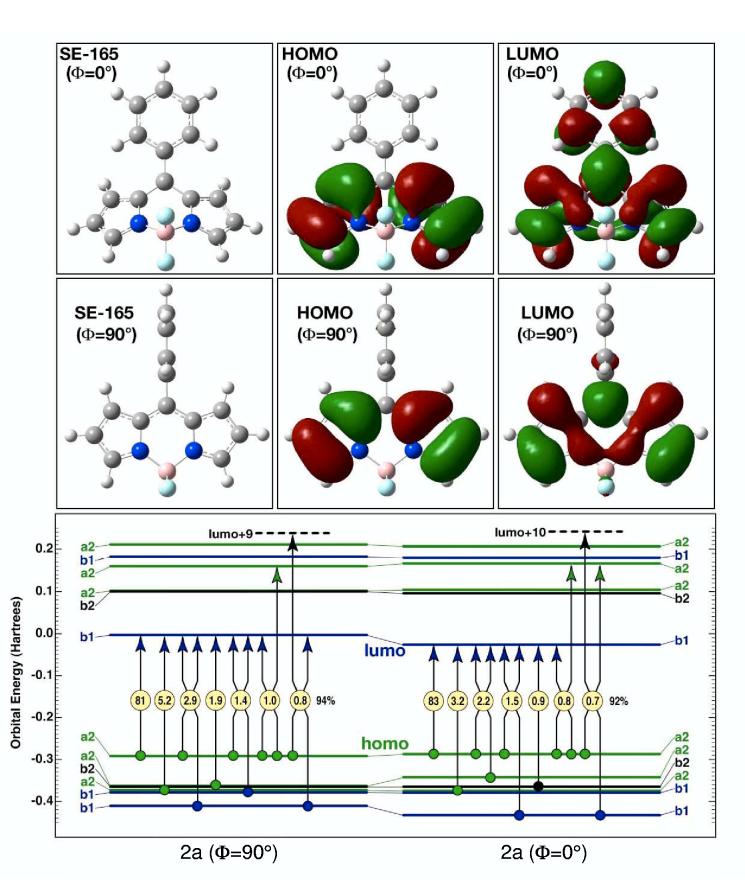
Figure 8.
The primary configurational properties of the lowest-lying B2 singlet states of 2a for two geometries of the phenyl ring: orthogonal to the boron-dipyrrin plane (Φ = 90°, left) and parallel to the dipyrrin plane (Φ = 0°, right). The lowest excited singlet state is highly ionic in character and is well described as a simple HOMO (a2) → LUMO (b1) one-electron excitation. The nature of the highest occupied molecular orbital (HOMO, a2 or a2-like symmetry) is relatively invariant in both character and energy to phenyl rotation. In contrast, the lowest unoccupied molecular orbital (LUMO, b1 or b1-like symmetry) extends into the phenyl group upon rotation and is highly stabilized by the increased extent of the pi-electron system when the phenyl group is parallel to the boron-dipyrrin plane (Φ = 0°). Stabilization of the LUMO is the dominant electronic driving force for phenyl group relaxation towards planarity in the S1 excited singlet state.