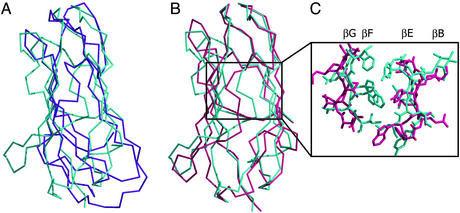
Figure 5.
Comparison of bNPC2 and dust mite allergen Der p 2. (A) Difference in the overall shape of bNPC2 and the NMR structure of Der p 2. A superposition of the Cα trace of bNPC2 (cyan) and the structure of Der p 2 determined by NMR (magenta) reveals different molecular envelopes of the two proteins, despite topologically similar folds. (B) Superposition of bNPC2 and the x-ray structure of Der p 2. The Cα traces of bNPC2 (cyan) and the crystal structure of Der p 2 (pink) are superimposed with an rms deviation of 2.9 Å. The two proteins align well at the top but deviate in the positioning of their β-sheets, with the two sheets in Der p 2 spaced more widely than those in bNPC2. (C) Comparison of the β-sheet interfaces in the crystal structures of bNPC2 and Der p 2. Central portions of the β-sheets are shown as Cα traces with side chains shown in stick representation. The β-sheets of bNPC2 (cyan) are more closely spaced and contain more bulky side chains than those of Der p 2 (pink). This difference explains the presence of a large tunnel in the crystal structure of Der p 2 that is absent in bNPC2.