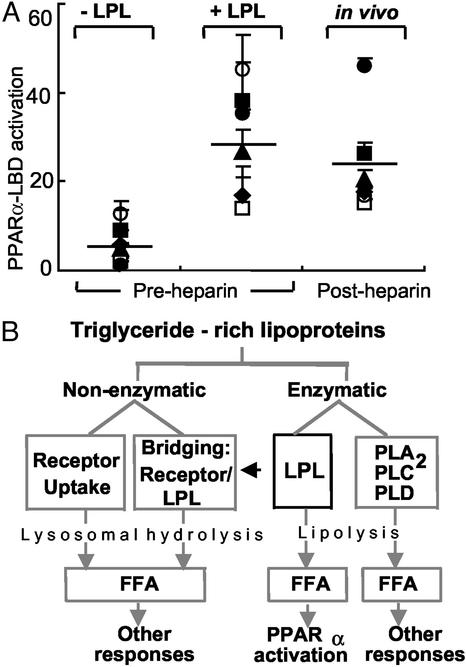
Figure 6.
LPL-mediated PPARα activation occurs as a unique component of triglyceride-rich lipoprotein metabolism. (A) PPARα-LBD transactivation assays were performed as in Fig. 2B by using human plasma samples (5%) obtained from six normolipidemic donors at baseline (−LPL), after addition of LPL in vitro (200 units/ml, 37°C, 1 h) to pre-heparin plasma, or after systemic heparin infusion (60 units/kg) in vivo. Symbols represent individual donors; lines indicate mean values. The relative PPARα-LBD fold activation for 10 μM WY14643 in this experiment was 20.3 ± 0.3; FA concentrations were 1.7 ± 0.8 μM (−LPL), 18.2 ± 9.3 μM (+LPL), and 18.2 ± 6.8 μM (post-heparin). (B) The metabolism of TRL occurs through both nonenzymatic and enzymatic mechanisms. Although these pathways ultimately result in FA production and their various downstream effects, PPARα activation through lipolysis seems restricted to some degree to LPL. The data presented suggest that specific mechanisms of endogenous lipolysis can lead to specific transcriptional responses through ligand generation. Thus, LPL-mediated PPARα activation may provide a link between circulating triglyceride-rich lipoproteins and distal PPARα effects such as antiinflammatory responses, FA oxidation, and lipid metabolism. The latter may include a possible positive feedback loop through PPARα leading to increased LPL expression and activity by inducing LPL and repressing ApoCIII.