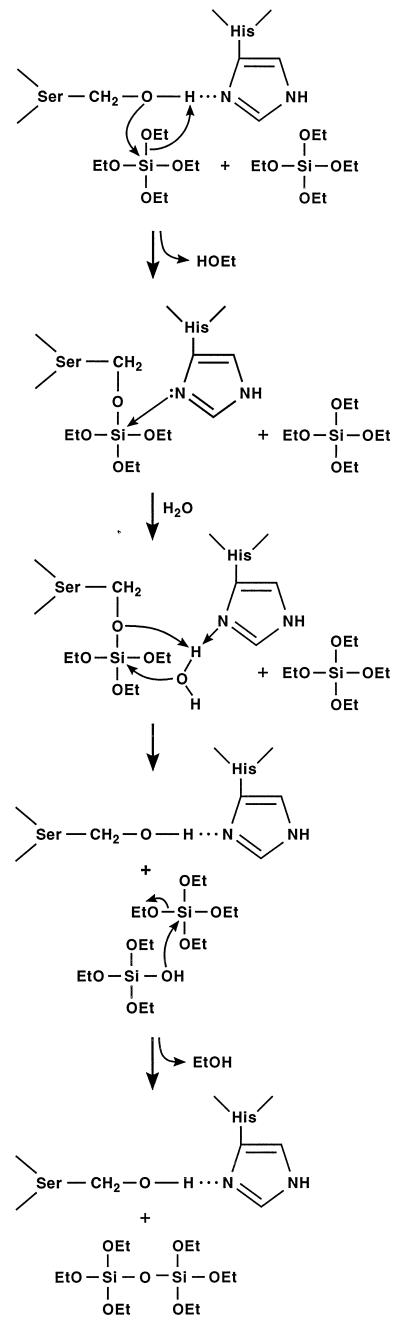
Figure 3.
Proposed reaction mechanism of silicon ethoxide condensation catalyzed by silicatein α, based on the well characterized mechanism of catalysis by the Ser/His and Cys/His active-site proteases (22). R = phenyl- or methyl- for the silicon triethoxide substrates, and R = CH3CH2—O— (= EtO—) for TEOS. Hydrogen-bonding between the imidazole nitrogen of the conserved histidine and the hydroxyl of the active-site serine is proposed to increase the nucleophilicity of the serine oxygen, potentiating its attack on the silicon atom of the substrate. Nucleophilic attack on the silicon displaces ethanol, forming a covalent protein—O—Si intermediate (potentially stabilized as the pentavalent silicon adduct via donor bond formation with the imidazole nitrogen). The addition of water completes hydrolysis of the first alkoxide bond. Condensation initiated by nucleophilic attack of the released Si—O− on the silicon of the second substrate molecule then forms the disiloxane product.