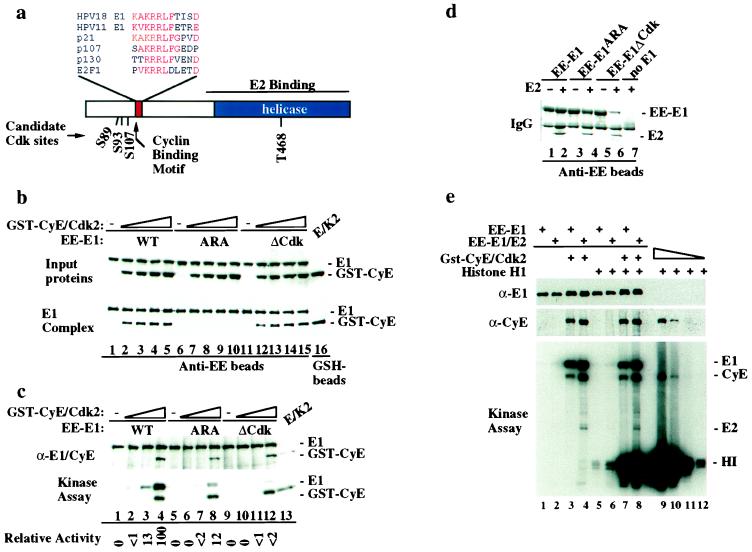
Figure 3.
An RXL motif in E1 is required for association with and phosphorylation by cyclin E/Cdk2. (a) Domains and positions of candidate Cdk phosphorylation sites in HPV E1 are shown. (b) Mutations in the RXL motif of E1 abolish association with cyclin E in vitro. Equal quantities (0.8 μg) of EE–E1 (lanes 1–5), EE–E1ARA (lanes 6–10), and EE–E1ΔCdk (lanes 11–15) were used for binding with increasing quantities of insect-cell lysates with or without cyclin E/Cdk2 and bound proteins detected by immunoblotting. To control for levels of input proteins, immunoblots of reaction mixtures prior to bead washing were performed (Top). (c) EE–E1 (lanes 1–4), EE–E1ARA (lanes 5–8), and EE–E1ΔCdk (lanes 9–12) immobilized on anti-EE beads were incubated with purified cyclin E/Cdk2 (1, 10, and 100 nM, respectively) and [γ-32P]ATP (see Materials and Methods). Products were separated by using SDS/PAGE and transferred to nitrocellulose before immunoblotting (Top) and autoradiography (Bottom). (d) E1ARA and E1ΔCdk interact with E2. Anti-EE immune complexes of insect-cell lysates derived from the indicated infections were separated by using SDS/PAGE and visualized with Coomassie blue. (e) Immobilized EE–E1 or EE–E1/E2 complexes were assembled with cyclin E/Cdk2 in vitro and the excess kinase removed before assay in the presence (lanes 5–8) or absence (lanes 1–4) of histone H1. As a control, serial dilutions of cyclin E/Cdk2 (1–1,000 ng) were used in histone H1 kinase assays (lanes 9–12).