Abstract
The binding of sphingoid bases to peroxisome proliferator-activated receptor (PPAR) has been detected in a solid-phase binding assay. However, sphingoid base–induced changes in PPAR transactivation activity have not been examined. In this report, we show by reporter gene analyses that phytosphingosine (PS), a natural sphingoid base, activates the transcriptional activity of PPARs in the immortalized human keratinocyte, HaCaT. Real-time PCR analyses showed that the mRNA level of PPARγ was increased after PS treatment in HaCaT cells in a dose- and time-dependent manner. Because PPARs play important roles in skin barrier homeostasis by regulating epidermal cell growth, terminal differentiation, and inflammatory response, we examined the effect of PS on normal human epidermal keratinocytes (NHEKs) and mouse skin. PS increased the production of cornified envelope in NHEKs by approximately 1.8-fold compared with controls. Epidermal differentiation marker proteins such as involucrin, loricrin, and keratin1 were also increased in PS-treated NHEKs, by ELISA or Western blotting analysis. A [3H]thymidine incorporation assay showed that PS inhibited DNA synthesis in NHEKs to 20% compared with controls. The antiproliferative and anti-inflammatory effects of PS were examined in a mouse model of irritant contact dermatitis produced by topical application of 12-O-tetradecanoylphorbol-13-acetate (TPA). PS blocked epidermal thickening and edema and the infiltration of inflammatory cells into the dermis in the skin of TPA-treated hairless mice. The anti-inflammatory effects of PS were confirmed by the observation that PS blocked the TPA-induced generation of prostaglandin E2 in peripheral mononuclear leukocytes. Taken together, our results provide an insight into the multiple regulatory roles of PS in epidermal homeostasis, and furthermore point to the potential use of PS as a therapeutic agent in the treatment of inflammatory and proliferative cutaneous diseases.
INTRODUCTION
Peroxisome proliferator-activated receptors (PPARs), which are members of the nuclear receptor family of ligand-activated transcription factors, control the transcription of target genes involved in many cellular functions, including lipid metabolism, cell proliferation, differentiation, and inflammatory responses. The 3 PPAR isoforms, PPARα, PPARβ/δ and PPARγ, have been identified in keratinocytes. PPARα and PPARγ are expressed at low levels but their levels increase dramatically during keratinocyte differentiation. In contrast, the predominant isotype, PPARβ/δ, is significantly induced under conditions of keratinocyte proliferation. Substantial evidence has accumulated that implicates PPARs in the regulation of epidermal differentiation and skin barrier homeostasis. Administration of PPARα activators was shown to stimulate differentiation and inhibit proliferation in rodent keratinocytes (1) and to promote development of the fetal epidermal permeability barrier (2). Recently, it was reported that topical treatment with PPAR agonists accelerates the differentiation and reverses the induced inflammatory hyperproliferation of murine epidermis (3,4). These results suggest that PPARs hold great promise for the therapy of skin conditions characterized by hyperproliferation, inflammatory infiltrates, and aberrant differentiation, such as psoriasis and atopic dermatitis (5)
Sphingolipids have been shown to be major structural components of stratum corneum (SC) intercellular lipid bilayers, which constitute the permeability barrier of mammalian skin (6). In addition, sphingolipids, including sphingoid bases and their metabolites, are suggested to act as intracellular signaling mediators in various biological functions of the mammalian epidermis, such as cell growth, differentiation, apoptosis, and inflammatory responses (7–10). Among sphingolipids, sphingosine is known for its anti-inflammatory and antiproliferative activities in mouse models of irritant contact dermatitis (7). These properties of sphingosine were partly attributed to its inhibitory activity on the PKC signaling pathway (7). Recently, during a search for endogenous ligands of PPARs, the binding of sphingoid bases to PPARα was studied in a solid-phase binding assay that employed recombinant mouse PPARα and a panel of different natural and synthetic lipids immobilized on silica layers. This report suggested that PPARs may be additional proteins that contribute to the overall biological activities of sphingoid bases (11).
Phytosphingosine (PS) is structurally similar to sphingosine, except that PS has a hydroxyl group at C-4 of the sphingoid long-chain base instead of a trans double bond between C-4 and C-5 (Figure 1). PS is abundant in fungi and plants (12), but is also found in mammalian epidermis (13). With the exception of a study of the lipid composition of the epidermis showing that psoriatic scales have reduced amounts of PS (14), few studies have been performed to evaluate the role of PS in the epidermis. Kim et al. (15,16) showed that the PS derivatives, N-acetyl PS and tetraacetyl PS, induced programmed cell death of an immortalized human keratinocyte cell line HaCaT and identified genes with altered expression in cells treated with PS derivatives. Little is known, however, about the biological function of PS itself in the epidermis.
Figure 1.
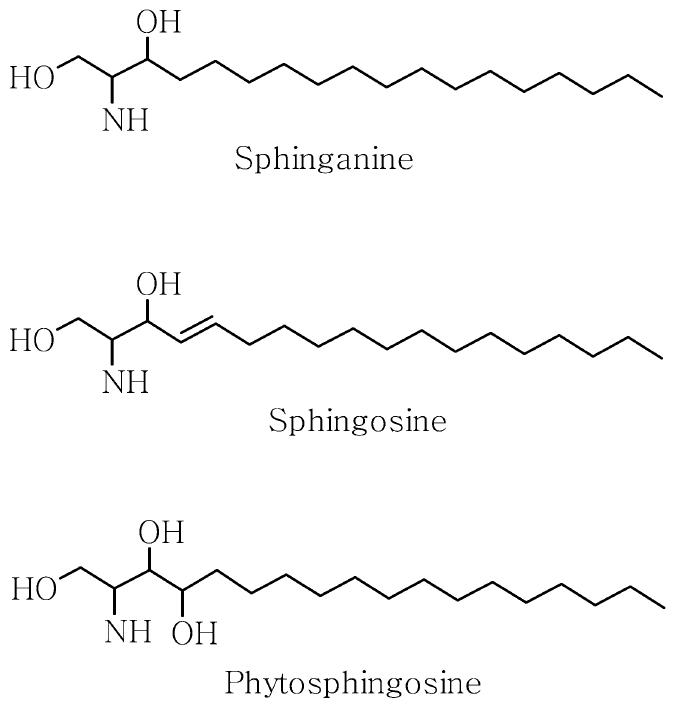
Structures of sphingoid bases.
In this study, we demonstrated that PS activated the transcriptional activity of PPARs and upregulated the expression of PPARγ gene in HaCaT cells. PS also inhibited cell growth and promoted epidermal differentiation in cultured normal human epidermal keratinocytes (NHEKs). Finally, the antiproliferative and anti-inflammatory effects of PS on TPA-induced inflammatory hyperplasia of mouse skin were investigated.
MATERIALS AND METHODS
Chemicals
PS was obtained from Doosan Biotech (Kyunggi, Korea). The purity of this compound was 98%. All other chemicals, unless otherwise indicated, were from Sigma Chemical (St. Louis, MO, USA).
Cell Culture and Treatment
The spontaneously transformed human keratinocyte cell line HaCaT was kindly provided by Dr. N.E. Fusenig (Deutsches Krebsforschungszentrum, Heidelberg, Germany) (17). The cells were grown in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum (FBS) and antibiotics (100 μg streptomycin and 100 IU penicillin per mL).
NHEKs were derived from neonatal foreskin as previously described (18). Briefly, epidermis was isolated from newborn foreskins by incubation in Dispase, and a suspension of keratinocytes was obtained by incubation in 10 mM EDTA and subsequent trypsinization. Cells thus obtained were cultured with serum-free keratinocyte growth medium (KGM) (Clonetics, San Diego, CA, USA) and used for experiments after 2 or 3 passages.
Peripheral mononuclear leukocytes were isolated using Ficoll-Paque Plus (Amersham, Arlington Heights, IL, USA). Diluted mouse blood (1:1 dilution with PBS) was loaded on Ficoll-Paque and centrifuged at 400g for 30 min. The cell layer was then collected and incubated in RPMI-1640 with 10% FBS at 37 °C.
For cell experiments, test compounds were dissolved in dimethylsulfoxide (DMSO) on the day of the experiment and diluted with serum-free medium to obtain the appropriate concentrations. The final volume of vehicle was adjusted to 0.1% (vol/vol). Control groups were treated with the same amount of vehicle.
Plasmids and Reporter Gene Assays
The reporter plasmid containing 3 PPAR response elements (PPREs), PPRE-tk-Luc, and the receptor expression plasmids, pCMX-mPPARs, have been described previously (19,20). A modified calcium phosphate precipitation procedure was used for transient transfection (21). Briefly, HaCaT cells (1 × 105 cells/well) were seeded into a 12-well culture plate and transfected with DNA mixtures (1.3 μg/well) containing carrier DNA (pBluescript), reporter plasmid (0.1 μg), and the β-galactosidase (β-gal) expression vector (0.2 μg) with or without pCMX-mPPAR expression vectors (10 ng), using LipofectaminePlus (Gibco BRL, Grand Island, NY, USA). The cells were treated for 24 h with test compounds. At the end of the incubation, luciferase activity was determined using a luminometer according to the manufacturer’s instructions. The luciferase activity was normalized for transfection efficiency using the corresponding β-gal activity.
RNA Preparation, Reverse Transcription, and Real-Time PCR
At the end of treatment, cells were washed twice with Dulbecco’s phosphate-buffered saline (PBS), and total RNA was isolated from cells using the Trizol reagent (Gibco BRL Life Technologies) according to the manufacturer’s instructions. Four μg total RNA was reverse-transcribed using MuLV reverse transcriptase (2.5 units), and the resulting cDNA was amplified by a thermal cycler (ICycler; Bio-Rad, Hercules, CA, USA) with rapid temperature control in a 50-μL reaction mixture containing AmpliTaq DNA polymerase (0.04 units) (Perkin Elmer, Shelton, CT, USA), 50 mM Tris (pH 8.3), 0.25 mg/mL bovine serum albumin, 3 mM MgCl2, 0.25 mM dNTPs, 1/50,000 dilution of SYBR Green I (Molecular Probes, Eugene, OR, USA), and 0.25 μM of the forward and reverse PCR primers. The primers used for PPARγ were forward, 5′-CAG ATC CAG TGG TTG CAG-3′, and reverse, 5′-GTC AGC GGA CTC TGG ATT-3′. Cycling conditions were as follows: one denaturing cycle at 95 °C for 5 min, followed by 30 cycles of 95 °C for 30 s, 53 °C for 30 s, and 72 °C for 1 min. Relative mRNA levels were determined by analyzing the changes in SYBR Green I fluorescence during PCR according to the manufacturer’s instructions with the ICycler software. To confirm that there was no fluorescence resulting from either genomic DNA contamination or reverse transcription, RNA (1 to 3 μg) without reverse transcriptase was analyzed in triplicate. Each PCR run also included triplicate wells of no template control where RNase-free water was added to reaction wells. β-Actin was amplified in parallel, and the results were used for normalization, as previously described (22). The size of PCR product was confirmed by electrophoresis on a 1% agarose gel stained with ethidium bromide. Purity of the amplified PCR products was determined by melting point analysis using ICycler software.
Quantitation of Cornified Envelope
Cornified envelope (CE) content of cultured keratinocytes was determined using a previously described method (23). Briefly, NHEKs were treated for 7 days with either CaCl2 or test compounds at the indicated concentrations and harvested in 2% SDS solution. After slight sonication (5 s), aliquots were removed for protein determination. The lysate was centrifuged at 12,000 rpm for 15 min and resuspended in 2% SDS/20 mM dithiothreitol (DTT) and boiled for 1 h, and the amounts of soluble cross-linked envelopes were quantified by spectrophotometry at 310 nm.
Involucrin Assay
The amount of involucrin in NHEK cultures was determined as previously described (24). Briefly, following 4 days of treatment with either CaCl2 or test compounds at the indicated concentrations, cells were harvested and homogenized. After microcentrifugation at 12,000 rpm for 15 min, supernatants were analyzed for involucrin using an enzyme-linked immunosorbent assay (ELISA) kit (Biomedical Technologies, Stoughton, MA, USA)
Western Blotting
At the end of the treatment with test compounds, NHEK cells were lysed in a lysis buffer consisting of 8 M urea, 2% CHAPS, 50 mM DTT, 2 M thiourea, 2 mM PMSF, 100 μg/mL leupeptine, and 25 μg/mL aprotinin. The lysate was then subjected to centrifugation at 15,000g for 10 min, and the supernatant was used for Western blot analysis. Protein concentrations were determined by the Bradford method using bovine serum albumin as the standard. Proteins (80 μg per well) were fractionated by SDS-PAGE and transferred to nitrocellulose membrane. The membrane was blocked with 5% skim milk in TBST (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.15% Tween-20) for 1 h at room temperature and subsequently probed overnight at 4°C with antiloricrin (1:500 dilution in TBST containing 5% skim milk) or anti-K1 antibodies (1:500 dilution in TBST containing 5% skim milk) (BabCo, Berkeley, CA, USA). Blots were washed 3 times with TBST and incubated with horseradish peroxidase–conjugated goat anti-rabbit IgG (Bio-Rad) at 1:1000 dilution at room temperature for 1 h. Detection was performed with the ECL system (Amersham Pharmacia Biotech, Piscataway, NJ, USA). The relative protein levels were analyzed using ImageMaster 2D Elite software (Amersham Biosciences, Buckinghamshire, UK). β-Actin was used as a loading control.
[3H]Thymidine Incorporation Assay
NHEKs grown to 70% confluence in 24-well plates were treated in triplicate with vehicle or test compounds for 24 h. Four hours before harvesting, cells were pulsed with 1 mCi/mL [3H]thymidine (2 Ci/mM; New England Nuclear, Boston, MA, USA). The medium was removed, and the cells were washed twice with ice-cold PBS. Cells were then incubated on ice with ice-cold 10% (wt/vol) trichloroacetic acid once for 10 min and then twice for 5 min to fix cells and precipitate DNA. The cells were lysed with 1 mL/well lysis buffer containing 0.3 N NaOH and 1% SDS for 15 min at room temperature. Cell lysates were transferred to scintillation vials containing 5 mL scintillation liquid and mixed well. [3H]thymidine radioactivity was measured, and the values were normalized with respect to the protein concentration.
Animal Treatment and Tissue Preparation
This study was conducted in conformity with the policies and procedures of the Institutional Animal Care and Use Committee of AmorePacific Corporation R&D Center. Hos:hr-1 albino male hairless mice and male ICR mice, purchased from Biogenomics (Seoul, Korea) at 7 weeks of age, were used for this study. The backs of hairless mice or the ears of ICR mice were treated with TPA (10 nmoles in acetone) and then divided into 3 groups (n = 7). Each group of mice was treated with vehicle (ethanol:propylene glycol, 7:3), PS (20 μmoles), or troglitazone (20 μmoles) for 30 min after TPA treatment. The hairless mice were killed 3 days after the single treatment, and 4-mm punch biopsies were taken and embedded in paraffin. Five cross-sections were taken from each tissue and stained with H&E. Stained slides were quantitatively characterized via digital image analysis using ImagePro-Plus (Media Cybernetics, Silver Spring, MD, USA). Images were captured through an Olympus BH-2 microscope fitted with a MicroImage video camera (Boyertown, PA, USA). Parameters such as area of epidermis and length of basement membrane were taken from a series of 10 random images on several slides to obtain a mean value for statistical comparison. Epidermal thickness was determined by the following formula: area of epidermis/length of basement membrane. Similarly, 4-mm full skin biopsies from the ears of ICR mice were taken for measurement of weight and myeloperoxidase (MPO) activity.
MPO Assay
MPO activity was determined using the supernatants from the homogenates of the ear biopsy (25,26). The tissues were placed in 1.5 mL of 50 mM sodium phosphate buffer, pH 6.0, containing 0.5% hexadecyltrimethylammonium bromide (HTAB) and homogenized for 45 s at 0 °C in a motor-driven homogenizer (Polytron PT 1200; Kinematica, Cincinnati, OH, USA). For the assay of MPO, we followed the method of Bradley et al. (26). Briefly, 50 μL supernatant, 50 μL phosphate buffer, pH 6.0, containing 0.5% HTAB, and 50 μL o-dianisidine solution (0.68 mg/mL in distilled water) were added into the wells of a 96-well microtiter plate, and the reaction was started by adding 50 μL freshly prepared 0.003% hydrogen peroxide. The optical density at 450 nm was read immediately and thereafter at 5-min intervals. The enzyme activity in the samples was obtained by comparing the rate of the reaction with that in wells containing supernatant from the control group treated only with TPA.
Prostaglandin E2 Assay
Mouse peripheral mononuclear leukocytes were cultured in 96-well plates (2 × 105 cells/well). After preincubation with TPA (16 nM) for 2 h, the cells were treated with either the cyclooxygenase inhibitor, indomethacin (1 nM and 10 nM), PS (5μM), or troglitazone (TGZ, 5 μM) for 16 h. The prostaglandin E2 (PGE2) concentration was measured using an enzyme immuno-assay (EIA) kit (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer’s instructions.
Statistics
The ANOVA test was performed using SigmaStat (SPSS, Chicago, IL, USA). The results were considered significant at P < 0.05.
RESULTS
Phytosphingosine Increased the Transcriptional Activity of PPARs and the Expression of PPARγ Gene
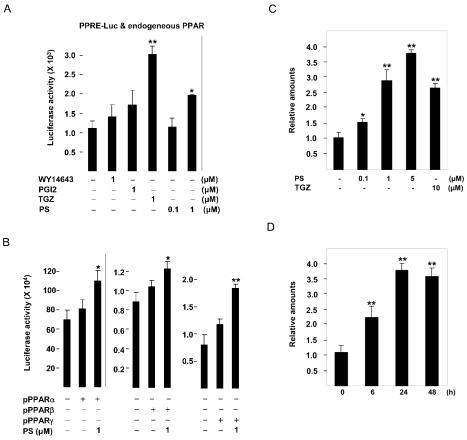
We tested whether known PPAR ligands and PS could activate the transcriptional activity of endogenous PPARs in HaCaT cells using transient transfection experiments employing the PPRE-tk-Luc reporter gene containing 3 PPAR response elements (19). When PPRE-tk-Luc was transfected into HaCaT cells, the synthetic PPARγ ligand troglitazone (TGZ) and PS at 1 μM induced reporter activity by approximately 3.0- and 2.0-fold, respectively, whereas the PPARα activator WY-14643 and the putative PPARβ/δ agonist prostacyclin (PGI2) had no effect (Figure 2A). When the reporter gene was cotransfected with PPARα, PPARβ/δ, and PPARγ expression vectors, PS at 1 μM increased reporter activity by approximately 36%, 17%, and 57%, respectively (Figure 2B). These results suggested that PPARγ acts as a dominantly active PPAR isoform in HaCaT cells and that PS can increase the transcriptional activity of PPARs with or without ectopic expression of PPAR isotypes.
Figure 2.
The effect of phytosphingosine on the transcriptional activity of PPARs and the expression of PPARγ gene. PPRE-tk-Luc was transiently transfected (A) or cotransfected with 10 ng of the pCMX-mPPAR expression vector into HaCaT cells (B) as described in “Materials and Methods.” Transfected cells were treated with WY14643, prostacyclin (PGI2), troglitazone (TGZ), or phytosphingosine (PS) as indicated and assayed 24 h later for luciferase activity. The corresponding β-gal activity was used to normalize luciferase activity. HaCaT cells were treated with indicated concentrations of phytosphingosine (PS) or 10 μM troglitazone (TGZ) for 24 h (C) or treated with 5 μM PS for the indicated time periods (D). At the end of incubation, total RNA was isolated and mRNA levels of PPARγ were measured by quantitative real-time RT-PCR analysis as described in “Materials and Methods.” The mRNA level of PPARγ in vehicle control or control at 0 h was set at 1, and the relative expression levels are presented as fold induction compared with that of each control. Data represent the mean ± SD of 3 or 4 independent experiments. *Significant at P < 0.05. **Significant at P < 0.01.
Next, we examined the effect of PS on the expression of PPARγ gene, because previous studies showed that the activation of PPARγ by TGZ increased PPARγ gene expression in several cultured cell lines (27). As shown in Figure 2C and D, mRNA level of PPARγ was increased after PS treatment in HaCaT cells in a dose- and time-dependent manner. A maximum of 3.8-fold was obtained with 5 μM concentration after 24-h treatment (Figure 2C,D).
Phytosphingosine Inhibited the Proliferation and Stimulated the Differentiation of Human Epidermal Keratinocytes
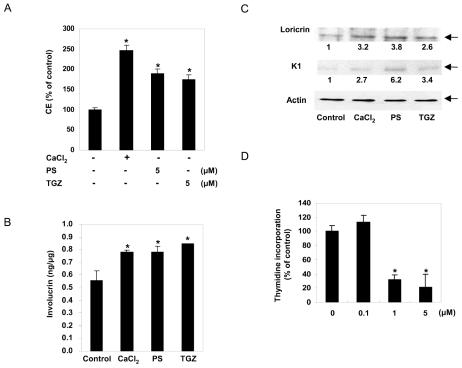
When proliferating keratinocytes are induced to differentiate in vitro by treating with PPAR activators or by changing the calcium ion concentration in the culture medium, the expression of differentiation-specific proteins, such as involucrin, loricrin, and K1, is induced and the amount of insoluble CEs is increased (1,28). To determine the effect of PS on keratinocyte differentiation, NHEK cells grown in low-calcium medium (0.05 mM CaCl2) were shifted to high-calcium medium (1.5 mM CaCl2) or treated with TGZ or PS, and cultured for an additional 4 or 7 days before harvesting. As shown in Figure 3A, treatment of keratinocytes with 5 μM PS for 7 days increased the production of CE by approximately 1.9-fold, which was similar to that seen with TGZ. When examined by ELISA, the level of involucrin increased by about 40% after exposure to 5 μM PS for 4 days (Figure 3B). The level of involucrin in keratinocytes treated with PS was comparable to that of cells cultured in high-calcium medium. The levels of loricrin and K1 as measured by Western blotting analysis were also significantly increased by treatment with 5 μM PS (Figure 3C).
Figure 3.
The effect of phytosphingosine on the proliferation and differentiation of keratinocytes. (A) For the measurement of cornified envelope (CE) content, normal human epidermal keratinocytes were treated for 7 days with Ca2+ (1.5 mM), phytosphingosine (PS), or troglitazone (TGZ) at the concentrations indicated. CEs were extracted by exhaustive boiling followed by sonication and quantification by spectrophotometry at 310 nm. Data are means ± SD of 3 independent experiments. *Significant at P < 0.05. (B and C) To measure epidermal differentiation marker proteins, normal human epidermal keratinocytes were treated for 4 days with high Ca2+ (1.5 mM), 5 μM phytosphingosine (PS), or 5 μM troglitazone (TGZ). The level of involucrin was analyzed by ELISA. Data are means ± SD of 3 independent experiments. *Significant at P < 0.05. The expression of loricrin and keratin1 was assessed by immunoblotting with antiloricrin or antikeratin1 antibody. The relative densitometric ratios are indicated. The data are representative of 3 independent blots. (D) Normal human epidermal keratinocytes in 24-well plates were treated with the indicated concentrations of phytosphingosine (PS). [3H]thymidine (1 μCi) was added to the wells 20 h after the addition of PS, the plates were incubated for 4 h, and the amount of cell-associated radioactivity was quantified. The indicated DNA synthesis levels are the means of the levels of triplicate wells ± SD expressed as a percent of the control. The DNA synthesis rate for the control was 1,570,072 ± 141,322 cpm/well. *Significant at P < 0.01.
The differentiation of keratinocytes is often accompanied by the inhibition of proliferation. To determine whether PS has an inhibitory effect on keratinocyte growth, the rate of DNA synthesis was measured by the [3H]thymidine incorporation assay. PS at 5 μM decreased [3H]thymidine incorporation into DNA by 80%, compared with the control (Figure 3D). The antiproliferative effect of PS was not due to cytotoxicity—the MTT assay and microscopic evaluation of keratinocytes treated with PS at a concentration of 5 μM did not reveal any signs of cytotoxicity (data not shown).
These results demonstrate that PS induces the differentiation and inhibits proliferation of cultured human keratinocytes.
Phytosphingosine Inhibited TPA-Induced Hyperproliferation and Inflammation in Mouse Skin
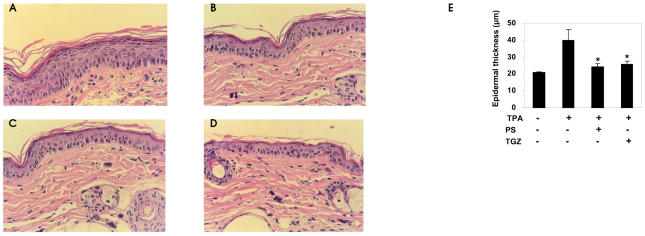
Antiproliferative and prodifferentiating effects of PPAR activators have been evaluated in a murine model of irritant contact dermatitis, where epidermal hyperplasia by topical treatment with TPA reproduced some of the abnormalities of several common skin disorders (29). Topical treatment with PPAR agonists has been shown to result in a marked decrease in epidermal hyperplasia and a reduction in the number of inflammatory cells in the dermis. In the same murine model, we assessed the antiproliferative and anti-inflammatory effects of PS. First, we examined H&E-stained sections of the back skins from TPA-treated hairless mice. As shown in Figure 4A and E, the epidermal thickness of the hairless mouse skin increased by about 2-fold 3 days after TPA treatment. In animals treated with TPA together with PS or TGZ, the epidermis was thinner than in animals treated with TPA alone (Figure 4B,C,E). Similarly, PS reduced the TPA-induced increase in the ear weight of ICR mice (Figure 5). The MPO activity of TPA-treated ear samples was also strongly reduced by cotreatment with PS (Figure 5), indicating that PS has strong inhibitory effects on the cellular migration of polymorphonuclear leukocytes. Taken together, these results indicate that PS treatment inhibits epidermal hyperplasia and inflammation by a magnitude similar to that seen with TGZ.
Figure 4.
Inhibition of TPA-induced hyperplasia by phytosphingosine (H&E staining, ×400). Hairless mice were topically treated with TPA (10 nmoles in acetone) and then divided into 3 groups (n = 7). Each group of mice was treated with (A) vehicle (ethanol:propylene glycol, 7:3), (B) phytosphingosine (PS, 20 μmoles), or (C) troglitazone (20 μmoles) 30 min after TPA treatment. Three days after a single application, 4-mm punch biopsies were taken and stained with H&E. (D) Control group. (E) The epidermal thickness was determined using a computer-assisted digital morphometric analyzer, ImagePro-Plus software. Data are presented as means ± SEM (n = 7). *Significant at P < 0.05.
Figure 5.
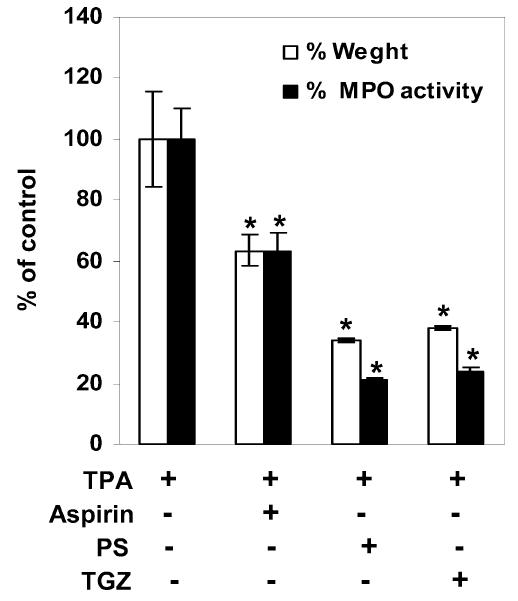
Inhibition of TPA-induced edema and MPO activity by phytosphingosine. The inner and outer surfaces of ears of ICR mice were treated with TPA (10 nmoles in acetone) and then vehicle (ethanol:propylene glycol, 7:3), aspirin (20 μmoles), phytosphingosine (PS, 20 μmoles), or troglitazone (TGZ, 20 μmoles). Edema was evaluated by measuring the weight of ears, and MPO activity was measured by the dianisidine-H2O2 assay. Data are presented as means ± SEM (n = 7). *Significant at P < 0.05.
Phytosphingosine Reduced TPA-Induced PGE2 Secretion from Mononuclear Leukocytes
We next examined whether PS affects on TPA-induced production of the inflammatory mediator, PGE2, which is known as to modulate the production of proinflammatory cytokines and to sustain local and systemic inflammatory responses (30,31). As shown in Figure 6, TPA induced a significant increase in PGE2 production. PS at 5 μM inhibited PGE2 production by 38%.
Figure 6.
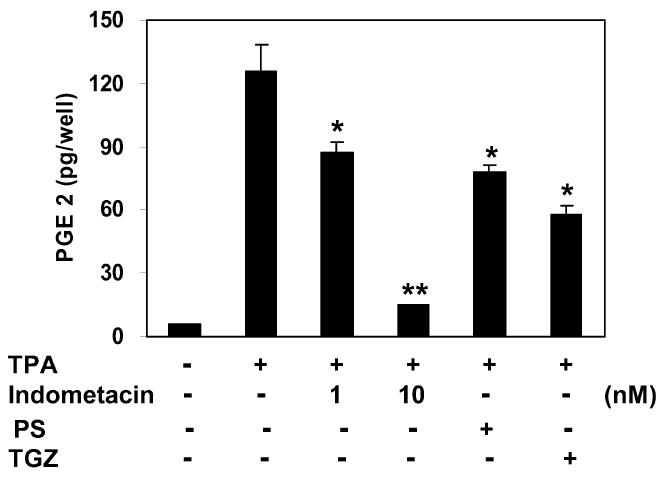
Inhibition of TPA-induced PGE2 synthesis by phytosphingosine. Mouse peripheral mononuclear leukocytes were treated with TPA (16 nM) and indomethacin (1 nM and 10 nM), phytosphingosine (PS, 5 μM), or troglitazone (TGZ, 5μ M) for 16 h. PGE2 released into culture media was analyzed by enzyme immuno-assay. Data represent the mean ± SD of 3 independent experiments. *Significant at P < 0.05. **Significant at P < 0.01.
DISCUSSION
The activities of sphingolipids as signaling modulators in the epidermis have been studied extensively. In spite of their structural similarity to sphingosine, however, the physiological roles of PS in the epidermis are still largely unknown. To date, PS has been shown to induce apoptosis in human cancer cells (32,33). Considering that terminal differentiation of keratinocytes has been suggested to be a specialized form of apoptosis (34,35), one action of exogenously supplied PS on keratinocytes might be the induction of the cellular and molecular events that are shared by keratinocyte differentiation and apoptosis. From this point of view, we tried to elucidate the regulatory roles of PS in keratinocyte differentiation and skin barrier homeostasis.
First, we examined whether PS activates the transcriptional activity of PPARs in immortalized human keratinocyte HaCaT cells using transient transfection experiments. Previously, the interaction between sphingoid bases and PPARα in a solid-phase binding assay suggested that PPARs might be proteins that contribute to the overall biological activity of sphingoid bases (11). Furthermore, the PPAR family is one of the most extensively investigated nuclear receptor families and has been implicated in the maintenance of epidermal homeostasis. We found that PS activated the transcriptional activity of PPARs in HaCaT cells to an extent comparable to that of the synthetic PPARγ ligand TGZ. Because the activation of PPARγ results in upregulation of PPARγ gene expression (27), we examined the effect of PS on the expression of PPARγ gene. Real-time PCR analyses showed that the mRNA level of PPARγ was increased after PS treatment in HaCaT cells in a dose- and time-dependent manner.
Next, the effect of PS on the differentiation of cultured NHEKs was examined. Because CE, involucrin, loricrin, and K1 are well-characterized markers of keratinocyte differentiation (23,36,37), we examined whether these markers were increased by PS treatment. As expected, PS treatment caused significant increases in CE formation and in involucrin, loricrin, and K1 protein levels in NHEKs cultured in low-calcium, serum-free medium. Keratinocyte differentiation induced by PS was accompanied by growth inhibition, as determined by the [3H]thymidine incorporation assay. These results demonstrate that PS promotes differentiation and inhibits the proliferation of NHEKs in vitro.
We also investigated the antiproliferative and anti-inflammatory effects of PS on TPA-induced inflammation and epidermal hyperproliferation in mouse skin, because PPAR activators have been shown to be potent inhibitors of TPA-induced inflammatory hyperplasia responses (29). The histological alterations induced by TPA in mouse skin were similar to those reported in other studies (7,38). TPA treatment resulted in a marked increase in the epidermal thickness of hairless mouse skin. The ear weight of ICR mice was also markedly increased following TPA treatment. Treatment with PS greatly reduced the magnitude of TPA-induced increases in epidermal thickness and ear weight. MPO activity, which indicates the infiltration of polymorphonuclear leukocytes into skin, was increased in TPA-treated ears but dramatically reduced in ears cotreated with TPA and PS. Furstenberger and Marks (39) reported that the level of PGE2 increased shortly after topical TPA treatment and that this early increase in PGE2 content appeared to be an important mediator of the pleiotypic response to TPA in the mouse skin. To examine whether PS interfered with TPA by inhibiting PGE2 synthesis, the effect of PS on the TPA-induced PGE2 synthesis in mouse peripheral mononuclear leukocytes was examined in cell culture. PGE2 production by TPA was blocked dramatically by treatment with PS. These results indicate that PS is a potent inhibitor of TPA-induced inflammatory hyperplasia.
In summary, we have shown that PS activates the transcriptional activity of PPARs and subsequently promotes the differentiation and growth arrest of keratinocytes in vitro. In addition, PS prevents TPA-induced inflammatory hyperplasia in hairless mouse skin. Our results suggest that PS treatment could be of a therapeutic benefit in a variety of inflammatory and hyperproliferative cutaneous diseases.
ACKNOWLEDGMENTS
We thank Dr. R. M. Evans for providing the PPRE-tk-Luc plasmid. The research of Dr. Jae Sung Hwang was supported in part by a grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (A050432).
Footnotes
Online address: http://molmed.org
REFERENCES
- 1.Hanley K, et al. Keratinocyte differentiation is stimulated by activators of the nuclear hormone receptor PPARα. J Invest Dermatol. 1998;110:368–75. doi: 10.1046/j.1523-1747.1998.00139.x. [DOI] [PubMed] [Google Scholar]
- 2.Hanley K, et al. Fetal epidermal differentiation and barrier development in vivo is accelerated by nuclear hormone receptor activators. J Invest Dermatol. 1999;113:788–95. doi: 10.1046/j.1523-1747.1999.00743.x. [DOI] [PubMed] [Google Scholar]
- 3.Komuves LG, et al. Keratinocyte differentiation in hyperproliferative epidermis: topical application of PPARα activators restores tissue homeostasis. J Invest Dermatol. 2000;115:361–7. doi: 10.1046/j.1523-1747.2000.00076.x. [DOI] [PubMed] [Google Scholar]
- 4.Komuves LG, et al. Stimulation of PPARα promotes epidermal keratinocyte differentiation in vivo. J Invest Dermatol. 2000;115:353–60. doi: 10.1046/j.1523-1747.2000.00073.x. [DOI] [PubMed] [Google Scholar]
- 5.Kuenzli S, Saurat J-H. Peroxisome proliferator-activated receptors in cutaneous biology. Br J Dermatol. 2003;149:229–36. doi: 10.1046/j.1365-2133.2003.05532.x. [DOI] [PubMed] [Google Scholar]
- 6.Gray GM, White RJ. Glycosphingolipids and ceramides in human and pig epidermis. J Invest Dermatol. 1978;70:336–41. doi: 10.1111/1523-1747.ep12543527. [DOI] [PubMed] [Google Scholar]
- 7.Gupta AK, Fisher GJ, Elder JT, Nickoloff BJ, Voorhees JJ. Sphingosine inhibits phorbol ester-induced inflammation, ornithine decarboxylase activity, and activation of protein kinase C in mouse skin. J Invest Dermatol. 1988;91:486–91. doi: 10.1111/1523-1747.ep12476635. [DOI] [PubMed] [Google Scholar]
- 8.Wakita H, et al. Keratinocyte differentiation is induced by cell-permeant ceramides and its proliferation is promoted by sphingosine. Arch Dermatol Res. 1994;286:350–4. doi: 10.1007/BF00402228. [DOI] [PubMed] [Google Scholar]
- 9.Geilen CWT, Orfanos C. Ceramide signaling: regulatory role in cell proliferation, differentiation and apoptosis in human epidermis. Arch Dermatol Res. 1997;289:559–66. doi: 10.1007/s004030050240. [DOI] [PubMed] [Google Scholar]
- 10.Di Nardo A, et al. Ceramide 2 (N-acetyl sphingosine) is associated with reduction in Bcl-2 protein levels by Western blotting and with apoptosis in cultured human keratinocytes. Br J Dermatol. 2000;143:491–7. doi: 10.1111/j.1365-2133.2000.03700.x. [DOI] [PubMed] [Google Scholar]
- 11.Van Veldhoven PP, Mannaerts GP, Declercq P, Baes M. Do sphingoid bases interact with the peroxisome proliferator activated receptor? (PPAR-α)? Cell Signal. 2000;12:475–9. doi: 10.1016/s0898-6568(00)00092-9. [DOI] [PubMed] [Google Scholar]
- 12.Dickson RC. Sphingolipid functions in Saccharomyces cerevisiae: comparison to mammals. Annu Rev Biochem. 1998;67:27–48. doi: 10.1146/annurev.biochem.67.1.27. [DOI] [PubMed] [Google Scholar]
- 13.Schurer NY, Plewig G, Elias PM. Stratum corneum lipid function. Dermatologica. 1991;183:77–94. doi: 10.1159/000247644. [DOI] [PubMed] [Google Scholar]
- 14.Motta S, et al. Ceramide composition of the psoriatic scale. Biochim Biophys Acta. 1993;1182:147–51. doi: 10.1016/0925-4439(93)90135-n. [DOI] [PubMed] [Google Scholar]
- 15.Kim HJ, Kim HJ, Lim SC, Kim SH, Kim TY. Induction of apoptosis and expression of cell cycle regulatory proteins in response to a phytosphingosine derivative in HaCaT human keratinocyte cells. Mol Cells. 2003;16:331–7. [PubMed] [Google Scholar]
- 16.Kim HJ, Shin W, Park CS, Kim HO, Kim TY. Differential regulation of cyclooxygenase-2 expression by phytosphingosine derivatives, NAPS and TAPS, and its role in the NAPS or TAPS-mediated apoptosis. J Invest Dermatol. 2003;121:1126–34. doi: 10.1046/j.1523-1747.2003.12554.x. [DOI] [PubMed] [Google Scholar]
- 17.Boukamp P, et al. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–71. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibson DFC, Ratnam AV, Bikle DD. Evidence for separate control mechanisms in the message, protein, and enzyme activation levels for transglutaminase during calcium-induced differentiation of normal and transformed human keratinocytes. J Invest Dermatol. 1996;106:154–61. doi: 10.1111/1523-1747.ep12329856. [DOI] [PubMed] [Google Scholar]
- 19.Kliewer SE, et al. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci U S A. 1994;91:7355–9. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee M-O, Kang HJ. Role of coactivators and corepressors in the induction of the RARbeta gene in human colon cancer cells. Biol Pharm Bull. 2000;25:1298–1302. doi: 10.1248/bpb.25.1298. [DOI] [PubMed] [Google Scholar]
- 21.Lee M-O, Kang HJ, Kim Y, Oum J-H, Park J. Repression of FasL expression by retinoic acid involves a novel mechanism of inhibition of transactivation function of the nuclear factors of activated T-cells. Eur J Biochem. 2002;269:1162–70. doi: 10.1046/j.1432-1033.2002.02748.x. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Pillai S, Cho S, Mahajan M, Frew L, Rawlings AV. Synergy between vitamin D precursor 25-hydroxyvitamin D and short chain ceramides on keratinocyte proliferation and differentiation. J Invest Dermatol Symp Proc. 1996;1:39–43. [PubMed] [Google Scholar]
- 24.Piazza GA, Ritter JL, Baracka CA. Lyso-phosphatidic acid induction of transforming growth factors β and γ: modulation of proliferation and differentiation in cultured human keratinocytes and mouse skin. Exp Cell Res. 1995;216:51–64. doi: 10.1006/excr.1995.1007. [DOI] [PubMed] [Google Scholar]
- 25.de Young LM, Kheifets JB, Ballaron SJ, Young JM. Edema and cell infiltration in the phorbol ester-treated mouse ear are temporally modulated by pharmacologic agents. Agents Action. 1989;26:335–41. doi: 10.1007/BF01967298. [DOI] [PubMed] [Google Scholar]
- 26.Bradley PP, Prieb DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–9. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 27.Li M, et al. Activation of peroxisome proliferator-activated receptor-gamma by troglitazone (TGZ) inhibits human lung cell growth. J Cell Biochem. 2005;96:760–74. doi: 10.1002/jcb.20474. [DOI] [PubMed] [Google Scholar]
- 28.Hennings H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980;19:245–54. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- 29.Sheu MY. Topical peroxisome proliferator activated receptor-γ activators reduce inflammation in irritant and allergic contact dermatitis models. J Invest Dermatol. 2002;118:94–101. doi: 10.1046/j.0022-202x.2001.01626.x. [DOI] [PubMed] [Google Scholar]
- 30.Janabi N, Chabrier S, Tardieu M. Endogenous nitric oxide activates prostaglandin F2 alpha production in human microglial cells but not in astrocytes: a study of interactions between eicosanoids, nitric oxide, and superoxide anion (O2−) regulatory pathways. J Immunol. 1996;157:2129–35. [PubMed] [Google Scholar]
- 31.Minghetti L, et al. Interferon-gamma and nitric oxide down-regulate lipopolysaccharide-induced prostanoid production in cultured rat microglia cells by inhibiting cyclooxygenase-2 expression. J Neurochem. 1996;66:1963–70. doi: 10.1046/j.1471-4159.1996.66051963.x. [DOI] [PubMed] [Google Scholar]
- 32.Park MT, et al. Phytosphingosine induces apoptotic cell death via caspase 8 activation and Bax translocation in human cancer cells. Clin Cancer Res. 2003;9:878–85. [PubMed] [Google Scholar]
- 33.Nagahara Y, et al. Phytosphingosine induced mitochondria-involved apoptosis. Cancer Sci. 2005;96:83–92. doi: 10.1111/j.1349-7006.2005.00012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haake AR, Polakowska RR. Cell death by apoptosis in epidermal biology. J Invest Dermatol. 1993;101:107–12. doi: 10.1111/1523-1747.ep12363594. [DOI] [PubMed] [Google Scholar]
- 35.Eleonora C, Rainer S, Gerry M. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6:328–40. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- 36.Dlugosz AA, Yuspa SH. Coordinate changes in gene expression which mark the spinous to granular cell transition in epidermis are regulated by protein kinase C. J Cell Biol. 1993;120:217–25. doi: 10.1083/jcb.120.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckert RL, Crish JF, Robinson NA. The epidermal keratinocyte as a model for the study of gene regulation and cell differentiation. Physiol Rev. 1997;77:397–424. doi: 10.1152/physrev.1997.77.2.397. [DOI] [PubMed] [Google Scholar]
- 38.Gupta AK, et al. Topical cyclosporine A inhibits the phorbol ester induced hyperplastic inflammatory response but not protein kinase C activation in mouse epidermis. J Invest Dermatol. 1989;93:379–86. [PubMed] [Google Scholar]
- 39.Furstenberger G, Marks F. Early prostaglandin E synthesis is an obligatory event in the induction of cell proliferation in mouse epidermis in vivo by the phorbol ester TPA. Biochem Biophys Res Commun. 1980;92:749–56. doi: 10.1016/0006-291x(80)90767-6. [DOI] [PubMed] [Google Scholar]