Abstract
Objective
To determine the effects of the anabolic agent oxandrolone on muscle protein and gene expression in severely burned children.
Summary Background Data
The authors previously showed that oxandrolone increased net muscle protein synthesis in emaciated burned patients receiving delayed treatment for severe burns. They hypothesized that similar effects would be seen in those treated early after burn.
Methods
Thirty-two severely burned children were enrolled in a prospective randomized trial. Subjects underwent studies to assess leg protein net balance 5 days after the first excision and grafting procedure. Immediately after these studies, treatment with placebo (n = 18) or 0.1 mg/kg oxandrolone (n = 14) twice a day was started. One week after this, another net balance study was performed in each subject. Body weights and total body potassium counting were used to determine body compositional changes. Muscle biopsies were taken 1 week after treatment in oxandrolone subjects to examine gene expression changes with gene array (12,600 genes).
Results
Protein net balance did not change in the placebo group, while oxandrolone-treated subjects had a significant improvement. Body weights and fat free mass significantly decreased in the placebo group, while no changes were found in the oxandrolone-treated subjects. Expression changes were seen in 14 genes in the oxandrolone group compared to placebo. Some of these included myosin light chain (+2.7-fold change), tubulin (+2.3), calmodulin (−2.3), and protein phosphatase I inhibitor (−2.8).
Conclusions
Oxandrolone improves protein net balance and lean mass in the severely burned. These changes are associated with increased gene expression for functional muscle proteins.
Severe injuries produce profound hypermetabolic stress responses that are characterized in part by protein catabolism, severe loss of lean body mass, and muscle wasting. 1–3 These changes contribute to increased morbidity and mortality and prolonged recovery from injury. The results of hypermetabolism persist for weeks to months, depending on the severity of the insult. 3 In the past, clinicians addressed loss of lean mass during the acute hospitalization with nutritional support. Administration of sufficient calories to support the hypermetabolic response to injury has been shown to reduce weight loss in severely burned patients 4 and to decrease loss of body mass in other critically ill patients. 5 Although weight loss can be diminished in severe catabolic states through nutritional support, severely injured patients still undergo massive wasting of the peripheral musculature. 6,7 Therefore, investigations into treatments in addition to provision of diet to reduce injury-induced muscle catabolism are required to fully overcome breakdown of lean body mass and thus improve outcomes.
Massive burns of more than 40% total body surface area (TBSA) cause severe protein catabolism and are an excellent model to study the effects of injury on protein metabolism. Hormones released with the endocrine response to stress drive this metabolic change. 8,9 Protein is mobilized to provide amino acids for energy as well as building blocks for host defense protein synthesis. Since no pool of stored protein exists, most of the required amino acids are taken from active muscle tissue, thus the loss of lean mass.
A number of anabolic hormones have been used effectively to abrogate lean mass catabolism in patients after severe burns when given over a portion of the hospital stay. These include insulin, 10–12 growth hormone, 13–15 insulinlike growth factor I, 16–18 oxandrolone, 19,20 and testosterone. 21 Of these, oxandrolone is an excellent candidate to increase net protein synthesis during hospitalization after severe injury for several reasons: 1) it is less expensive than growth hormone and insulinlike growth factor, thus providing a fiscal benefit, 2) it is administered orally, alleviating the need for intravenous access or intramuscular injections, and 3) its side effect profile is well described, with substantially less virilization potential than other androgenic steroids such as testosterone.
Oxandrolone, an analog of testosterone, has been used clinically in adults to treat muscle wasting in AIDS wasting myopathy 22,23 and chronic obstructive pulmonary disease 24 and during convalescence from severe burn. 25,26 In children, it has been used for growth disorders associated with Turner syndrome 27 and constitutional growth delay. 28
We previously showed that oxandrolone was effective at increasing net protein synthesis in severely burned children who were emaciated from delays in treatment with inadequate nutritional delivery. In that study, we found that oxandrolone, when given for 1 week during the hospitalization, improved the net balance of amino acids across the leg, which was associated with increased protein synthesis and increased protein synthetic efficiency. No effects on protein breakdown were found. 20 In the present study, we sought to extend these findings to severely burned subjects who received early definitive treatment. We also sought to determine the effects of prolonged administration of oxandrolone throughout the hospital course on body compositional changes, and lastly, to determine the effects of oxandrolone on gene expression associated with physiologic improvements.
METHODS
This study was performed under a University of Texas Medical Branch Institutional Review Board-approved protocol. Informed written consent was obtained from each patient’s parent or guardian before enrollment into the study. Inclusion criteria were as follows: children less than 18 years of age, TBSA burns of greater than 30%, and had received definitive care continued at the Shriners Hospitals for Children within 2 weeks of injury who underwent at least two wound closure operations and thus were able to complete two stable isotopic studies.
Patient Care
Within 48 hours of admission, each patient underwent total burn wound excision and grafting with autograft skin and allograft. Patients returned to the operating room when autograft donor sites healed and became available for reharvest (usually 6–8 days from the last operation). Sequential staged surgical procedures for repeat excision and grafting were undertaken until the wounds were healed. Each patient received enteral nutrition via a naso-duodenal tube with Vivonex TEN (Sandoz Nutritional Corp., Minneapolis, MN). The composition of Vivonex is 82% carbohydrate, 15% protein, and 3% fat. Daily caloric intake was given at a rate calculated to deliver 1,500 kcal/m2 TBSA burned + 1,500 kcal/m2 TBSA. This feeding regimen was started at admission and continued at a constant rate until the wounds were healed. Caloric intake remained constant throughout the hospitalization. Insulin was given by continuous infusion to keep the serum glucose level below 200 mg/dL, in accordance with standard accepted clinical practice. Insulin doses during the stable isotopic studies were recorded and compared between groups.
Patients were intubated for operations, after which extubation was accomplished as soon as possible. Ventilator settings for those who remained intubated followed ARDS-NET recommendations. 29 Sepsis, as previously defined, 7 was recorded and compared between groups. Subjects were at bed rest after excision and grafting procedures for 5 days. After this, patients ambulated daily until the next excision and grafting procedure. Patients were treated in an identical fashion in terms of mobilization and rehabilitation.
Study Design
We sought to determine whether oxandrolone treatment during the acute hospitalization would improve protein kinetics across the leg and body mass composition with associated gene expression changes. Between January 1999 and December 2001, 32 children meeting the study inclusion criteria were recruited. These subjects were not consecutive due to competing study enrollment. Assignment to these competing studies was conducted using a randomization schedule obtained with a random number generator. All subjects underwent metabolic evaluation, including weight, stable isotopic analysis of the balance of protein across the leg, and body composition analysis, approximately 7 days after acute admission. This was chosen as the baseline study point to minimize disruptive changes associated with resuscitation edema and the initial excision and grafting procedure. After these studies, subjects were randomized to receive either placebo or 0.1 mg/kg oxandrolone (BTG Pharmaceuticals, Iselin, NJ) administered orally or via feeding tube in an alcohol suspension twice a day for the rest of the hospitalization. Placebo was a matching tablet or suspension prepared by the research pharmacist at the Shriners Hospital. Based on power analysis, we targeted enrollment to 30 subjects. Treatment was blinded to clinical care staff and to those analyzing the data.
Approximately 1 week after instituting the study treatment, subjects underwent stable isotopic studies to assess the groups for differences. All stable isotopic studies were performed in the fed state. Treatment was continued until discharge, when another set of body compositional studies was obtained. Seven subjects from the oxandrolone group underwent percutaneous quadriceps muscle biopsy for gene array expression analysis.
Stable Isotope Studies
The degree of protein catabolism was quantified using stable isotope tracers. Protein kinetic studies were performed beginning between 5 and 7 am, on approximately postoperative day 5 after the first excision and grafting procedure. All stable isotope studies consisted of a 5-hour infusion of d5-phenylalanine (Cambridge Isotopes, Andover, MA), as previously described. 7 The initial priming dose of 2 μmol/kg was followed by a dose of 0.08 μmol/kg/min given intravenously. Femoral arteriovenous sampling during the fifth hour measured cross-leg phenylalanine balance. Blood samples were taken simultaneously from an ipsilateral femoral artery and vein for this determination. Some subjects (four in the placebo group and three in the oxandrolone group) underwent 8-hour studies with the administration of intravenous amino acids for hours 5 through 8. No significant changes were seen with amino acid administration; therefore, amino acid concentration and enrichment values were averaged to reach a single subject value. Indocyanine green (ICG) was used to determine leg blood flow. 30 ICG dye concentration was measured between hours 3 and 4 to determine leg blood flow. Muscle biopsies for DNA microarray analysis were snap-frozen and stored at −70°C.
The blood concentration of unlabeled phenylalanine was determined by gas chromatography-mass spectrometry (GCMS) using the internal standard approach and the nitrogen-acetol-n-propyl esters, as previously described. 31 The isotopic enrichment of free amino acids in blood was determined on a HP model 5989 (Hewlett-Packard Co., Palo Alto, CA) by chemical ionization and selected ion monitoring at mass-to-charge ratios of 250:1, 255:1, 256:1. ICG concentrations were determined spectrophotometrically at λ = 805 mm on a Spectronic 1001 (Bausch & Lomb, Rochester, NY).
As phenylalanine is neither synthesized nor degraded in the periphery, the difference in concentration of this substrate in the femoral arterial and venous plasma pools reflects the net balance of leg skeletal muscle protein synthesis and breakdown. The net balance (NB) was calculated and standardized for leg volume by the following equation:MATH
where CA and CV are the blood free amino acid concentrations of the femoral artery and vein and BF is leg blood flow in cc/min/100 mL leg. Leg blood flow was determined from the following modification of Fick’s equation, MATH
where CF is the femoral venous concentration of ICG and CC is the central (contralateral femoral) venous concentration of ICG. With the infusion rate set at 0.5 mg/min, the equation was solved for leg blood flow (BF). As indicated above, BF was normalized for each patient by leg volume. Subject weight, leg circumference at prescribed points relative to anatomic landmarks, and the distances between these points were used to mathematically model leg volume.
Two-Pool Protein Kinetic Model
The rates of appearance (Ra) and disappearance (Rd) of phenylalanine in the femoral arterial and venous plasma pools reflect leg skeletal muscle protein breakdown and synthesis, respectively. The phenylalanine kinetic rates within the leg were calculated and standardized for leg volume. MATH MATH MATH
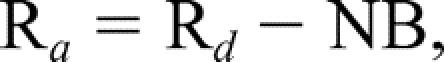 |
where NB is the net balance of protein synthesis and breakdown across the leg; Rd and Ra are the rates of disappearance and appearance of substrate within the leg, respectively; CA and CV are the blood free amino acid concentrations of the femoral artery and vein; EA and EV are amino acid enrichments (tracer/tracee ratio) in the femoral artery and vein; and BF is leg blood flow in cc/min/100 mL leg.
Body Composition Measures
Total weights were measured approximately 5 days after admission and at discharge for all subjects using standard clinical sling scales. Six-month weights were measured for subjects in both groups using standing scales. These clinical scales are calibrated monthly.
Total body lean mass and bone mineral content were measured by dual-energy x-ray absorptiometry. A Hologic model QDR-4500W DEXA (Hologic Inc., Waltham, MA) was used to measure body composition in 1999. This machine was replaced in 2000 when nonfunctional by a QDR-4500A Absorptiometer (Hologic). To minimize systematic deviations, the Hologic system was calibrated daily against a spinal phantom in the anteroposterior, lateral, and single-beam modes. Individual pixels were calibrated against a tissue bar phantom to determine whether the pixel was reading bone, fat, lean tissue, or air.
We were unable to obtain DEXA scans early in the hospital course in 13 of the 32 subjects because of dysfunction of the DEXA scanner or clinical instability precluding transport to the scanner room. For similar reasons, we were unable to obtain DEXA scan results for 10 subjects at the time of discharge. Eighteen subjects did not have DEXA scans at 6-month follow-up.
Fat free mass was determined by whole body potassium 40 (40K) scintillation counting in a heavily shielded low-background noise counting room, a 32-NaI detector array, and computed data analysis that has been validated for use in children. 32 The counting precision of the instrument used is less than 1.5%, which was calibrated daily using BOMAB phantoms with simulated fat overlays. All studies were done after feedings and intravenous fluids were discontinued to minimize exogenous potassium contamination. We were able to obtain seven such studies in placebo subjects and four in oxandrolone subjects early in the hospital course (within the first week of admission). We were able to get 12 in the placebo group and 10 in the oxandrolone group at discharge. Nine subjects in the placebo group and seven in the oxandrolone group underwent this study 6 months after injury.
Data and Statistical Analysis
Data are presented as means ± standard error of the mean or percent incidence as required. Continuous data within groups were compared by paired t test, and between groups by unpaired t test. Noncontinuous data were compared by Fisher exact tests. All values outside three standard deviations from the group mean were excluded from analysis (jack-knife procedure). For stable isotopic data, studies with negative values for Ra and Rd were eliminated from analysis, as this is physiologically impossible.
Gene Expression Analysis
Total RNA Extraction
Total RNA was isolated from muscle biopsies by acid guanidinium thiocyanate-phenol-chloroform extraction using TRI Reagent (Molecular Research Center Inc., Cincinnati, OH). This method was based on the single-step method of RNA isolation described by Chomczynski and Sacchi. 33 Samples were homogenized in TRI Reagent on ice and total RNA was extracted following the manufacturers’ instructions. Purified RNA was quantified by UV absorbance at 260 and 280 nm and stored in 25-μg aliquots at −70°C for DNA microarray hybridization and analyses. The adequacy and integrity of the extracted RNA were determined by gel electrophoresis. Of seven oxandrolone-treated subjects undergoing biopsy for gene array analysis, only four yielded sufficient RNA in tissue for biopsy taken before treatment and after treatment. Those without sufficient RNA from both time periods were eliminated from further analysis.
High-Density Oligonucleotide Array Analysis
Probe labeling, hybridization, and image acquisition were done according to the standard Affymetrix protocol. Briefly, 25 μg purified, total RNA was transcribed into cRNA, purified, and used as templates for in vitro transcription of biotin-labeled antisense RNA. Biotinylated antisense RNA preparation was fragmented and placed in a hybridization mixture containing four biotinylated hybridization controls (BioB, BioC, BioD, and Cre). Samples were then hybridized to an identical lot of Affymetrix Gene Chip arrays (HG-U95 Av2) for 16 hours. The arrays were washed and stained using the instrument’s standard Eukaryotic GE Wash 2″ protocol and antibody-mediated signal amplification. The images were scanned and analyzed with Affymetrix GeneChip Analysis Suite 3.2. Images from each Gene Chip were scaled and adjusted to an average intensity value for all arrays of 1,500. Scaled average difference values and absolute call data from each Gene Chip were exported to data files and used for statistical analysis. 34 In vitro transcription and chip hybridization were performed in collaboration with the UTMB Genomic Core Facility.
Gene Array Data Analysis
Data analysis of genomic data included chip validation, cluster analysis of transcription profiles, identification of genes expressed, and temporal analysis of gene expression at different time points after treatment. The first step in the analysis was to cluster the data to detect gross discrepancies among array data. The degree of similarity or dissimilarity among sample transcription profiles was tested using model-based expression analysis of oligonucleotide arrays. 34 The next step was the elimination of genes that showed little variation across the samples, or that were absent in the majority of the samples. The first criterion was that the ratio of standard deviation and the mean of a gene’s expression values across all samples was greater than the threshold (of 0.85 and the upper limit of 8). Data were discarded if there was a large deviation in the number of present calls or if the correlation coefficient among samples within the group was less than 0.85. The second criterion required a gene to be called present in more than 80% of arrays at all times. We determined the presence (P) or absence (A) of each probe within the group (according to the Affymetrix algorithm). A probe is “present” if its absolute call was “P” for at least two members of the group containing four samples. Otherwise, the gene was considered not expressed in the group. The primary goal was to identify genes with significant differences in expression in the oxandrolone-treated subjects before and after treatment. The average of expression for pre- and posttreatment was calculated and comparisons were made by computing the expression fold difference for each gene and listing those that show larger than two-fold increase or decreases in activity. An entry was discarded as an outlier when its value was outside 3 standard deviations. Only the statistically significant differences at P < .05 were retained. 35 Considering the number of samples per group and its influence on the validity of the analysis, the power of the t test was computed. If it was less than 0.8, results were discarded even when significant. The expression profiles of the skeletal muscle biopsies taken from oxandrolone-treated children were analyzed. Samples were taken at study 1 (baseline), before oxandrolone treatment, and study 2 was approximately 1 week after treatment began. By using HG-U95A Affymetrix arrays, about 4,000 genes of 12,000 genes present in the array were expressed. This was in agreement with Affymetrix DNA array analysis of mouse skeletal muscle. 36
RESULTS
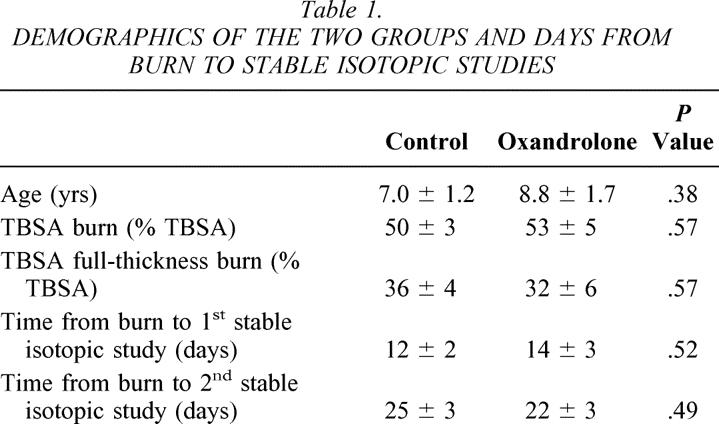
Demographics of the two groups are listed in Table 1. No significant differences were found in age, TBSA burned, percentage of burn that was full-thickness, or postburn days of study for the stable isotopic measures. The finding of no difference for days to stable isotopic studies indicates relative homogeny of physiologic status between groups for these measures. We also found no differences in insulin used clinically to control hyperglycemia between groups.
Table 1. DEMOGRAPHICS OF THE TWO GROUPS AND DAYS FROM BURN TO STABLE ISOTOPIC STUDIES
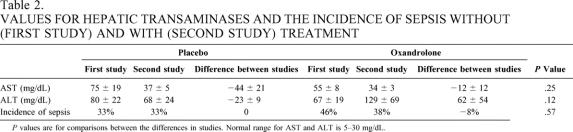
We assessed the groups for differences in reported common complications of oxandrolone treatment, which are virilization and elevation of hepatic transaminase levels. As a gross measure of immunocompetence, we determined the incidence of sepsis (Table 2). We found no evidence of virilization, such as the emergence of pubic hair, clitoral hypertrophy, or deepening of the voice in any of the children treated with either placebo or oxandrolone either at discharge or at 6 months of follow-up. We found no differences in hepatic transaminases between groups either during the first study or the second study. However, we found that AST and ALT levels decreased between the first study and the second study in the placebo group (P = .08 and P = .03 for AST and ALT, respectively), while they did not decrease in the oxandrolone group (P = .33 and P = .27, respectively). This implies that oxandrolone treatment prevented the normal decline in hepatic transaminases seen after severe burn in children. We found no differences in the incidence of sepsis between or within groups.
Table 2. VALUES FOR HEPATIC TRANSAMINASES AND THE INCIDENCE OF SEPSIS WITHOUT (FIRST STUDY) AND WITH (SECOND STUDY) TREATMENT
P values are for comparisons between the differences in studies. Normal range for AST and ALT is 5–30 mg/dL.
We also measured the length of hospital stay as a general determination of well-being and oxandrolone treatment. The length of hospital stay was 0.60 ± 0.05 days/% TBSA burn in the placebo group and 0.66 ± 0.09 days/% TBSA burn in the oxandrolone treatment group (P = .61).
Stable Isotope Studies
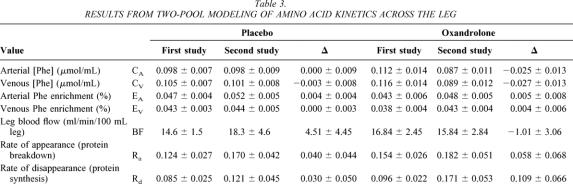
We performed stable isotopic studies with d5-phenylalanine tracer before and after treatment with placebo or oxandrolone to determine effects on amino acid kinetics in the muscle of severely burned children. We found that oxandrolone treatment was associated with a significant improvement in the net balance of amino acids across the leg, while similar improvements were not seen in those receiving placebo (Fig. 1). We previously demonstrated that this improvement with oxandrolone treatment is due to improved protein synthetic efficiency. 20 To assess the mechanism for net balance change, we performed a two-pool analysis of amino acid kinetics. We found that protein synthesis (Rd) seemed to increase with oxandrolone treatment, although not to a statistically significant value, presumably due to variability. Protein breakdown did not change (Table 3). These data are consistent with our previous data and demonstrate that oxandrolone improves the net balance of amino acids across the leg in severely burned children when given approximately 1 week after injury and receiving immediate definitive treatment.
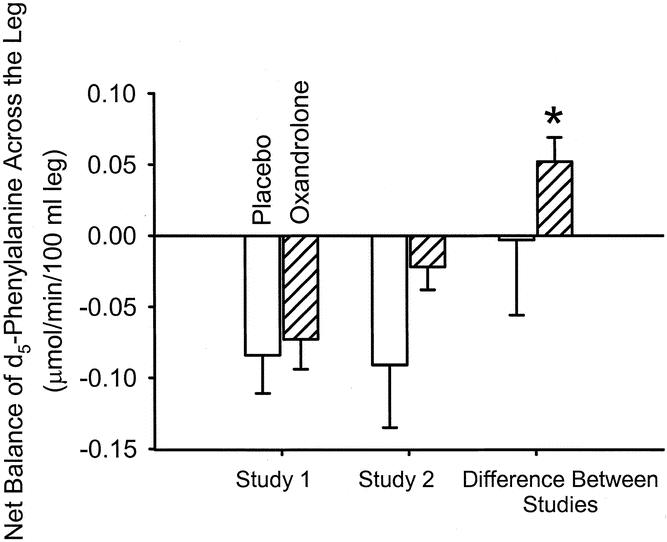
Figure 1. Net balance of d5-phenylalanine across the leg without and with oxandrolone treatment. Oxandrolone induced a significant improvement in net balance in the treated group (P = .01), while no significant difference was found in the placebo group.
Table 3. RESULTS FROM TWO-POOL MODELING OF AMINO ACID KINETICS ACROSS THE LEG
Whole Body Mass Measurements
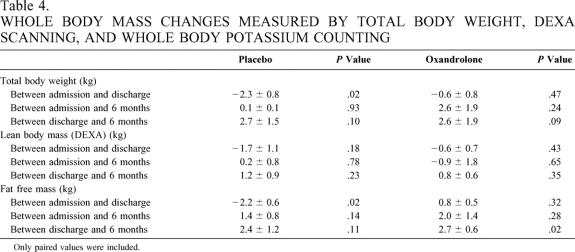
To determine whole body mass changes associated with oxandrolone treatment given throughout the hospitalization in severely burned children, we analyzed total body weights, lean body mass determined by DEXA scan, and fat free mass assessed by whole body potassium counting in children being treated with placebo or oxandrolone throughout hospitalization beginning approximately 1 week after admission. Body composition values were obtained close to admission after initial edema associated with resuscitation was eliminated and were compared to values obtained at discharge and 6 months after injury (Table 4).
Table 4. WHOLE BODY MASS CHANGES MEASURED BY TOTAL BODY WEIGHT, DEXA SCANNING, AND WHOLE BODY POTASSIUM COUNTING
Only paired values were included.
Weights at all time points were available for analysis (n = 18 for placebos and n = 14 for oxandrolone treatment). We found that total body weight significantly decreased in the subjects receiving placebo in a paired analysis (P = .02), while weight did not decrease in the oxandrolone-treated subjects (see Table 4). Dietary intake was not different between groups indexed to burn size.
No significant differences were found between groups at any time point in the DEXA analysis (see Table 4). Because of missing data points, six pairs of studies were available for both groups in the admission-versus-discharge analysis, seven pairs for placebo and three pairs for oxandrolone treatment in the discharge-versus-6-month analysis, and five pairs for placebo and four pairs for oxandrolone treatment in the admission-versus-6-month analysis.
Fat free mass determined by whole body potassium counting was significantly diminished in the placebo group between admission and discharge (P = .02), while no changes were seen in fat free mass in the oxandrolone-treated subjects. When comparing the changes in fat free mass during hospitalization between the placebo group and the oxandrolone group, a significant difference was also found (P = .02). Furthermore, fat free mass significantly increased in the treated group in the discharge-versus-6-month values (P = .02)(see Table 4). For the admission-versus-discharge analysis, five pairs were available for the placebo group and three pairs for the oxandrolone group. For the discharge-versus-6-month comparison, five pairs were available in the placebo group and four for the oxandrolone group. For the analysis of admission-versus-6-month values, five pairs were available in the placebo group and three in the oxandrolone group.
Gene Expression Changes
To assess alterations in gene expression associated with the outcome changes demonstrated above, we performed gene array analysis on muscle samples from four subjects receiving oxandrolone before and after treatment. We found significant changes in 14 genes with oxandrolone treatment (Table 5). Among those upregulated were the structural proteins myosin light chain, dynein, and tubulin. Downregulated genes included protein phosphatase I inhibitor, which is associated with inhibition of protein translation from mRNA. Other significant genes that were upregulated included those associated with the calcineurin/calmodulin/Ca++ signaling proteins. These proteins are linked with modulation of muscle hypertrophy and oxidative damage. 37,38
Table 5. GENE EXPRESSION CHANGES IN MUSCLE BIOPSY SPECIMENS FROM PATIENTS BEFORE AND AFTER OXANDROLONE TREATMENT
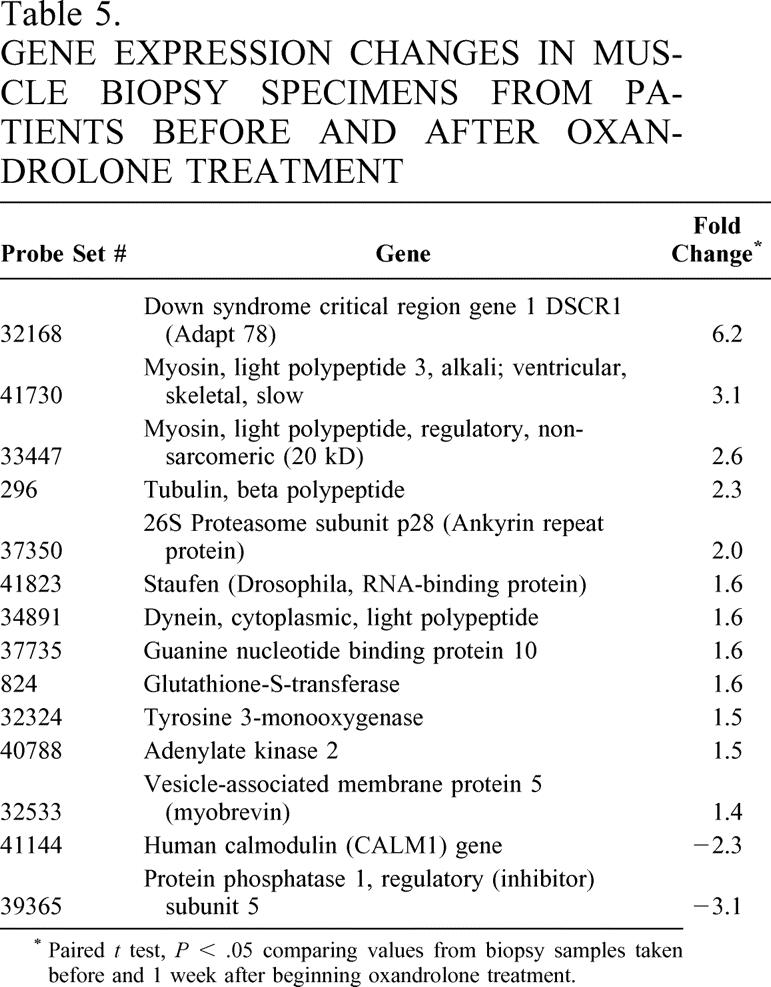
* Paired t test, P < .05 comparing values from biopsy samples taken before and 1 week after beginning oxandrolone treatment.
DISCUSSION
In this analysis, oxandrolone treatment started 1 week into hospitalization in severely burned children was associated with improved net balance of amino acids across the leg compared to those receiving placebo. When oxandrolone was continued throughout the rest of the hospitalization, it maintained body weight and fat free mass (muscle), while the placebo group lost weight and fat free mass. These physiologic changes were associated with gene expression changes for several structural proteins and those involved in the regulation of protein synthesis. These results indicate for the first time that oxandrolone treatment improves net muscle protein synthesis, which results in better fat free mass accretion when used following severe injury. It appears that the mechanisms responsible for these changes are related to changes in gene expression.
Reported complications of oxandrolone treatment include virilization, acne, glucose intolerance, and cholestatic jaundice and hepatitis. We analyzed the subjects in this study for the development of these complications. We found no incidence of virilization in any of the subjects nor any complaints of acne. The use of insulin to control hyperglycemia was not different between groups. However, we did find some potential differences in hepatic transaminases in those treated with placebo versus those treated with oxandrolone, implicating an effect of oxandrolone to induce chemical hepatitis when used in the severely injured. We found that the mean values for serum hepatic transaminases were above the normal range (5–30 mg/dL) at 1 and 2 weeks of hospitalization for both groups. However, the change in levels between week 1 and 2 was significantly different for the placebo group, while no change was seen in the oxandrolone group, suggesting that oxandrolone prevents the normal decline in hepatic transaminases following injury. Potential explanations include direct damage to hepatocytes or effects on metabolic pathways inducing hepatocyte death (indirect). Further studies in animal models will be necessary to find the mechanism of these changes. The clinical implications, however, appear to be minor, as we did not identify any instances of hepatic failure or dysfunction.
We found that oxandrolone treatment was associated with improved net protein balance across the leg. We previously showed that the effects of oxandrolone on muscle amino acid kinetics in normal adults and in children following severe burn is through a stimulation of muscle protein synthetic efficiency. 20,39 Stated more simply, oxandrolone seemed to stimulate protein synthesis such that intracellular amino acids resulting from protein breakdown are directed toward protein synthesis rather than transport out of the cell. Therefore, amino acids stay in the cell, thus improving protein accretion. We expect that the same mechanism is present in these subjects as well.
In addition to the effects we saw at the amino acid level, we found that oxandrolone treatment throughout the hospitalization was associated with improved weights and fat free mass in the oxandrolone-treated subjects. These data confirm the amino acid kinetic data above that indicate improved fat free mass accretion with oxandrolone treatment. In this analysis, we did not find any changes in lean body mass measured by DEXA. We can only speculate that this may be due to more variability in DEXA measurement of lean mass compared to total body potassium counting in the severely burned. In fact, this issue has not been systematically studied in severely burned children. We are currently performing both analyses in our ongoing studies until this question is answered.
We found several gene expression changes in association with the physiologic changes measured above, some of them very surprising. Among them was a dramatic upregulation of the Down syndrome candidate region I gene (Adapt 78). This gene was independently discovered as a resident of the “Down’s syndrome candidate region” and as an “adaptive response” stress gene that is transiently induced during oxidative stress. 40 Recently the DSCR1 (Adapt 78) gene product was discovered to be a potent inhibitor of calcineurin, a serine/threonine phosphatase, and its signaling pathways. DSCR1, a product of chromosome 21, 37,38 is highly expressed in brain, heart, skeletal muscle and is overexpressed in the brain of Down syndrome fetuses. It interacts physically and functionally with calcineurin A, the catalytic subunit of the Ca2+/calmodulin-dependent protein phosphatase PP2B. Overexpression of DSCR1 and ZAKI-4 inhibits calcineurin-dependent gene transcription through the inhibition of NF-AT (IL-6) translocation to the nucleus. In skeletal muscles, calcineurin signaling is implicated both in hypertrophic growth stimulated by IGF-1 37,41 and in the control of specialized programs of gene expression that establish distinctive myofiber subtypes and differentiation. 42 The finding of increased expression in the face of improved muscle protein synthesis is surprising; however, it can perhaps be explained through loss of feedback inhibition with oxandrolone stimulation of protein synthesis within the muscle. We are designing studies to determine whether this is the case.
In all eukaryotes, myosin plays a major role in the maintenance of cell shape and in cellular movement; in association with actin and other contractile proteins, it is also a major structural component of the muscle sarcomere. Several isoforms of myosin alkali light chain have been identified, associated with different muscle types, and play an important role in muscle function. Here, we found stimulation in gene expression for two myosin subtypes, in keeping with the finding of increased muscle mass in association with oxandrolone treatment. In addition, we found increased expression in other abundant structural proteins important in muscle function, including tubulin and dynein.
We found that p28, one of the subunits of the 26S proteasome, was significantly upregulated with oxandrolone treatment. Ubiquitin and the 26S proteasome are principal components of the major proteolytic system that is responsible for degrading a wide variety of intracellular proteins, including constitutively unstable regulatory proteins and proteins with aberrant structures generated by mutations or various environmental stresses. 43,44 These and other cellular proteins are targeted for degradation by the 26S proteasome after they have been covalently attached to ubiquitin in the form of a poly-ubiquitin chain. The 26S proteasome is composed of two large multiprotein complexes: the 20S proteasome and PA700 (also known as 19S complex). The 26S proteasome, unlike the 20S proteasome, degrades ubiquitinated proteins in an ATP-dependent fashion. Although the molecular mechanism of protein degradation by the 26S proteasome is unknown, PA700 may regulate and promote the translocation of polypeptide substrates through the proteasome’s terminal rings to the active sites. Our finding of increased gene expression for a subunit of a protein associated with protein breakdown can again be explained by loss of feedback inhibition. This notion must be verified with further studies.
To our knowledge, this is the first report of gene array analysis in this subpopulation of the severely injured. We reported earlier on gene expression changes in response to oxandrolone in a group of burned patients with delayed presentation and nutritional emaciation, 45 with some important differences. In fact, none of the genes differentially expressed in that study were present here. We speculate that this is due to changes in physiology with emaciation. We hope that further studies will bear this out.
We used gene array analysis as a tool to find gene expression changes associated with measured physiologic outcomes. This analysis primarily revealed changes in gene expression of functional structural proteins but also identified changes in proteins in the calcineurin/calmodulin pathway, providing a target for further mechanistic research. We are actively studying the role of this pathway in maintenance of muscle mass following severe injury.
In summary, we have shown that oxandrolone treatment started 1 week after hospitalization in severely burned children is associated with improved net muscle protein synthesis and improved body composition. These changes are associated with novel gene expression differences, providing new targets for exploration of the mechanisms of muscle protein loss following severe injury and the effects of treatment.
Discussion
Dr. Basil A. Pruitt, Jr. (San Antonio, TX): Dr. Wolf has brought us another important contribution from Dr. Herndon’s extensive research program that has expanded our understanding of the hypermetabolic response to injury. They have extended their earlier observation that oxandrolone improved net protein balance in emaciated burned children received late after injury to show now that twice-a-day oxandrolone therapy can reduce lean body mass loss of patients admitted early after injury when metabolic rate is greatest. To interpret these findings and evaluate your conclusions, we need some additional information.
On page 6 of the manuscript you state that the feeding regimen continued at a constant rate until the wounds were healed. Since metabolic rate decreases as wound size decreases, did delayed healing of relatively small areas of the wounds of the treatment group result in overall greater calorie and protein loads being responsible for the preservation of body mass?
The full-thickness burns of the control group on average involved 4% more of the body surface. Did you make provision to weigh the tissue excised from each patient? If that was done, did a greater amount of excised tissue account for the greater weight loss in the control group?
You state on page 7 and page 15 of the manuscript that four patients in each group underwent muscle biopsy for gene expression analysis. But on page 10 you note that three of the treatment group biopsies and one control group biopsy couldn’t be analyzed. Does that mean that the comparison data displayed in Table 4 are based on a biopsy from a single treated patient? If that is so, do you feel comfortable with a comparison group having an N of 1?
Some of the changes in gene expression appear to be consistent with the observed increase in protein synthesis. Since you have previously shown that oxandrolone does not affect protein degradation rate, how do you explain the apparent insensitivity of the patients to the two-fold increase in p26 gene expression, that being a subunit of the p28 proteosome gene?
The data in Table 3 are also of concern. You note that oxandrolone therapy was associated with “improved weight and fat free mass.” However, lean body mass as assessed by DEXA scan was not different in the two groups. Is it possible that the difference in weight simply reflects greater water retention in the tissues of treatment-group patients? Does DEXA scanning differentiate water from other tissue components and correct for water content in determining lean body mass?
You found that oxandrolone prevented the “normal” postburn decline in hepatic transaminases. Was that associated with an increased hepatic steatosis? If so, can that be ameliorated by propranolol treatment, which you have previously reported to decrease hepatic fat uptake?
Lastly, since length of hospital stay was not different in the two groups, was there some other clinical benefit, such as earlier ambulation or more rapid recovery of some physical capabilities, that accrued from the use of oxandrolone?
Dr. William G. Cioffi, Jr. (Providence, RI): I would like to echo Dr. Pruitt’s congratulations to Drs. Wolf and Herndon on another paper helping to delineate the appropriate metabolic support for these massively thermally injured children.
Some of my questions are similar to Dr. Pruitt’s, and I would like more information on the clinical relevance of your findings. The statistical changes and cross-limb differences are one thing, but what does this mean clinically to the patient? As Dr. Pruitt mentioned, there was no change in length of stay. Do you have wound healing data as you did in your growth hormone experiments? Was there increased physical strength? Did these patients get through rehab quicker? Dr. Pruitt also talked about water retention, which is a known side effect of anabolic steroids. You did in the paper talk about your DEXA measurements, but you didn’t mention it in the oral presentation. You did talk about the potassium measurements. Can you exclude water retention?
My biggest concern is, you have now discussed growth hormone, insulinlike growth factor 1 with its binding protein 3, insulin, and now a synthetic derived testosterone derivative, and shown improvements in protein synthetic rates or degradation rates for each of them. So which of these should we use? What are the relative effects of each of them? Which is most effective clinically? What are the costs? And what are the untoward effects of each? How should we treat our patients?
Your gene chip data is interesting, but like most gene chip data, it doesn’t talk about functional proteins. Do you have any functional protein data saying the RNA changes you show really mean anything? As Dr. Pruitt alluded, some of the increases in RNA have to do with protein degradation, not synthesis.
Finally, in the paper there wasn’t any difference at 6 months. It appeared that at least in terms of weight, which was your main outcome measurement, at 6 months postinjury there was no difference. So what should you do? Should you treat these patients longer? Does it really matter at all if we use the testosterone derivative? Or should you use a different agent? I ask this question because of the known long-term detrimental effects of anabolic steroid use and wonder if 6, 12, or 18 months of therapy would be detrimental to the children.
Dr. George M. Watkins (Tampa, FF): This usual superb paper reminds me that I actually started medical school and worked in genetics for awhile, but concluded in 1958 there were seven medical geneticists in the U.S. and it never would be any good for a surgeon. We were dealing with the Inherited Disease File, as it was called in those days. But I have similar questions.
One of them is very easy, and I am sure was done clinically, and it is, for the people that were extubated, the ones that were intubated in both groups, negative respiratory pressure would be a very easy way to determine if there were any differences that were overt and obvious, and other pulmonary functions, if you had them, I think would be also. I agree with the mobilization itself.
About composition. It is not just protein; there are a number of things. You didn’t mention anything except potassium there, but you also have a number of electrolytes like calcium phosphate and so forth. Would you enlighten us a little on what the changes were there?
In the same regard, I know that you used to—I don’t know if you still do—used to measure the 24-hour creatinine clearances. And that gives a somewhat indirect, but a measure of how much creatinine in 24 hours by each person and each group was, and if you showed any significant difference in total creatinine excretion in the two groups, particularly during the acute phase.
And finally, I think maybe you have some 24-hour collection of the same thing, electrolytes—otherwise, the differences between the two groups. They may not be perfect, but they would help any answer any questions that may linger.
Dr. William C. Lineaweaver (Jackson, MS): I may not have been able to read all the details of the gene expression slide, but I wondered if you looked at any markers for expression of the TGF-beta isoforms. Certainly this cytokine can be associated with hypertrophic scarring. Parenthetically, were these patients examined for any evidence of hypertrophic scarring or contractures?
Dr. Steven E. Wolf (Galveston, TX): Dr. Pruitt asked what about our dietary program. All patients in both groups received the same relative amount of calories based on burn size. We arrived at our particular formula through the observation that this prescription (1,500 kcal/% TBSA burned in meters squared + 1,500 kcal/maintained total body weight in meters squared) maintained total body weight in a population of several hundred severely burned children. This prescription is and was given at the assigned rate throughout hospitalization in all of our patients. In regards to the possibility that the amount of tissue excised may have been different between groups, we did not measure this. We can only assume that this loss would be equally distributed between groups through randomization. No significant difference in burn size was detected between groups.
As far as gene expression, we did paired analyses. In other words, we obtained tissue for biopsy from a subject before and after treatment and determined differences between them. We previously showed that gene expression changes in muscle are minimal over the first few weeks after severe burn; therefore, it is reasonable to conclude that any changes that occur in an individual are due to treatment, in this case with oxandrolone.
We, with you, are a bit perplexed with the increase in mRNA expression for p28, an agent associated with ubiquitin-mediated protein breakdown. My only explanation at this time is that perhaps this is upregulated by loss of feedback inhibition associated with stimulated protein synthesis. This is of course purely speculative.
As far as the DEXA scans, we did not present that data here because no significant differences were found between groups. We feel the reasons for this may be due to fluid shifts that occur in the severely injured, increasing the variability of this measurement. For this reason, we relied instead on whole body potassium counting, which is more reliable as potassium is an intracellular ion that won’t be affected by interstitial fluid shifts.
As you know, hepatic steatosis is universal in the severely burned, and we are only now developing the tools to measure it in response to anabolic agents. We will report on the effects of oxandrolone on this in the future. Since we didn’t see any changes in length of stay, we are looking at other outcome measures such as strength and ability to rehabilitate, which might become evident in outpatient studies we are doing.
Dr. Cioffi, improvement in net balance of protein across the leg should correlate with improvements in muscle mass, as the muscle is the most metabolically active tissue in the leg. What we showed here was an improvement in net balance of amino acids across the leg, which was correlated with an increase in lean mass. What remains to be shown is whether the increase in lean mass constitutes an improvement in strength and the ability to rehabilitate more quickly. We are currently devising these measures. We did not measure wound healing in this study.
As far as the different anabolic agents that are available, and which should we use, I submit that oxandrolone is a pretty good one because it is orally administered, and it is relatively cheap, and appears to be as effective as anything else ($5 to $10/day as opposed to some injectables, which may be thousands of dollars/day).
The gene array analysis is only a beginning to dissecting the mechanisms of effect of oxandrolone on muscle. We plan to follow up on some of these changes in gene expression by doing protein analysis and protein activity assays, and fitting the changes into a signaling pathway.
Dr. Watkins mentioned a number of measures that we did not assess, including negative inspiratory force, serum calcium, phosphorus, and magnesium, and measures of renal function. We will look at these in future studies. As far as Dr. Lineaweaver’s comment, this gene array does have the TGF-beta isoforms on it, and we were unable to find any significant differences in comparison to the others.
References
- 1.Newsome TW, Mason AD Jr, Pruitt BA Jr. Weight loss following thermal injury. Ann Surg. 1973; 178: 215–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfe RR. Herman Award Lecture, relation of metabolic studies to clinical nutrition–the example of burn injury. Am J Clin Nutr. 1996; 64: 800–808. [DOI] [PubMed] [Google Scholar]
- 3.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000; 128: 312–319. [DOI] [PubMed] [Google Scholar]
- 4.Gore DC, Rutan RL, Hildreth M, et al. Comparison of resting energy expenditures and caloric intake in children with severe burns. J Burn Care Rehabil. 1990; 11: 400–404. [DOI] [PubMed] [Google Scholar]
- 5.Bower RH. Nutrition during critical illness and sepsis. New Horiz. 1993; 1: 348–352. [PubMed] [Google Scholar]
- 6.Streat SJ, Beddoe AH, Hill GL. Aggressive nutritional support does not prevent protein loss despite fat gain in septic intensive care patients. J Trauma. 1987; 27: 262–266. [DOI] [PubMed] [Google Scholar]
- 7.Hart DW, Wolf SE, Chinkes DL, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000; 232: 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bessey PQ, Watters JM, Aoki TT, et al. Combined hormonal infusion simulates the metabolic response to injury. Ann Surg. 1984; 200: 264–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gore DC, Jahoor F, Wolfe RR, et al. Acute response of human muscle protein to catabolic hormones. Ann Surg. 1993; 218: 679–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakurai Y, Aarsland A, Herndon DN, et al. Stimulation of muscle protein synthesis by long-term insulin infusion in severely burned patients. Ann Surg. 1995; 222: 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrando AA, Chinkes DL, Wolf SE, et al. A submaximal dose of insulin promotes net skeletal muscle protein synthesis in patients with severe burns. Ann Surg. 1999; 229: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas SJ, Morimoto K, Herndon DN, et al. The effect of prolonged euglycemic hyperinsulinemia on lean body mass after severe burn. Surgery. 2002; 132: 341–347. [DOI] [PubMed] [Google Scholar]
- 13.Gore DC, Honeycutt D, Jahoor F, et al. Effect of exogenous growth hormone on whole-body and isolated-limb protein kinetics in burned patients. Arch Surg. 1991; 126: 38–43. [DOI] [PubMed] [Google Scholar]
- 14.Knox J, Demling R, Wilmore D, et al. Increased survival after major thermal injury: the effect of growth hormone therapy in adults. J Trauma. 1995; 39: 526–530. [DOI] [PubMed] [Google Scholar]
- 15.Demling RH. Comparison of the anabolic effects and complications of human growth hormone and the testosterone analog, oxandrolone, after severe burn injury. Burns. 1999; 25: 215–221. [DOI] [PubMed] [Google Scholar]
- 16.Cioffi WG, Gore DC, Rue LW 3rd, et al. Insulin-like growth factor-1 lowers protein oxidation in patients with thermal injury. Ann Surg. 1994; 220: 310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herndon DN, Ramzy PI, DebRoy MA, et al. Muscle protein catabolism after severe burn: effects of IGF-1/IGFBP-3 treatment. Ann Surg. 1999; 229: 712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Debroy MA, Wolf SE, Zhang XJ, et al. Anabolic effects of insulin-like growth factor in combination with insulin-like growth factor binding protein-3 in severely burned adults. J Trauma. 1999; 47: 904–910. [DOI] [PubMed] [Google Scholar]
- 19.Demling RH, DeSanti L. Oxandrolone, an anabolic steroid, significantly increases the rate of weight gain in the recovery phase after major burns. J Trauma. 1997; 43: 47–51. [DOI] [PubMed] [Google Scholar]
- 20.Hart DW, Wolf SE, Ramzy PI, et al. Anabolic effects of oxandrolone after severe burn. Ann Surg. 2001; 233: 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrando AA, Sheffield-Moore M, Wolf SE, et al. Testosterone administration in severe burns ameliorates muscle catabolism. Crit Care Med. 2001; 29: 1936–1942. [DOI] [PubMed] [Google Scholar]
- 22.Berger JR, Pall L, Hall CD, et al. Oxandrolone and AIDS wasting myopathy. AIDS. 1996; 10: 1657–1662. [DOI] [PubMed] [Google Scholar]
- 23.Earthman CP, Reid PM, Harper IT, et al. Body cell mass repletion and improved quality of life in HIV-infected individuals receiving oxandrolone. JPEN. 2002; 26: 357–365. [DOI] [PubMed] [Google Scholar]
- 24.Yeh SS, DeGuzman B, Kramer T, M012 Study Group. Reversal of COPD-associated weight loss using the anabolic agent oxandrolone. Chest. 2002; 122: 421–428. [DOI] [PubMed] [Google Scholar]
- 25.Demling RH, Orgill DP. The anticatabolic and wound healing effects of the testosterone analog oxandrolone after severe burn injury. J Crit Care. 2000; 15: 12–17. [DOI] [PubMed] [Google Scholar]
- 26.Demling RH, DeSanti L. Oxandrolone, an anabolic steroid, significantly increases the rate of weight gain in the recovery phase after major burns. J Trauma. 1997; 43: 47–51. [DOI] [PubMed] [Google Scholar]
- 27.Stahnke N, Keller E, Landy H. Favorable final height outcome in girls with Ullrich-Turner syndrome treated with low-dose growth hormone together with oxandrolone despite starting treatment after 10 years of age. J Pediatr Endocrinol Metab. 2002; 15: 129–138. [DOI] [PubMed] [Google Scholar]
- 28.Wilson DM, McCauley E, Brown DR, et al. Oxandrolone therapy in constitutionally delayed growth and puberty. Pediatrics. 1995; 96: 1095–1100. [PubMed] [Google Scholar]
- 29.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000; 342: 1301–1308. [DOI] [PubMed] [Google Scholar]
- 30.Biolo G, Chinkes D, Zhang XJ, et al. Vars Research Award. A new model to determine in vivo the relationship between amino acid transmembrane transport and protein kinetics in muscle. JPEN. 1992; 16: 305–315. [DOI] [PubMed] [Google Scholar]
- 31.Biolo G, Maggi SP, Williams BD, et al. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol. 1995; 268: E514–520. [DOI] [PubMed] [Google Scholar]
- 32.Ellis KJ, Shypailo RJ. Total body potassium in the infant. J Radioanal Nucl Chem. 1992; 161: 61–69. [Google Scholar]
- 33.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987; 162: 156–159. [DOI] [PubMed] [Google Scholar]
- 34.Li C, Wong WH. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci USA. 2001; 98: 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dudoit S, Yang YH, Callow M, et al. Statistical methods for identifying differentially expressed genes in replicated cDNA microarray experiments. Department of Statistics, University of California at Berkeley, Technical report #578, 2000.
- 36.Lee CK, Klopp RG, Weindruch R, et al. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999; 285: 1390–1393. [DOI] [PubMed] [Google Scholar]
- 37.Semsarian C, Wu MJ, Ju YK, et al. Skeletal muscle hypertrophy is mediated by a Ca2+dependent calcineurin signalling pathway. Nature. 1999; 400: 576–581. [DOI] [PubMed] [Google Scholar]
- 38.Ernak G, Harris CD, Davies KJ. The DSCR1 (Adapt 78) isoform 1 protein calcipressin 1 inhibits calcineurin and protects against acute calcium mediated stress damage, including transient oxidative stress. FASEB J. 2002; 16: 814–824. [DOI] [PubMed] [Google Scholar]
- 39.Sheffield-Moore M, Urban RJ, Wolf SE, et al. Short-term oxandrolone administration stimulates net muscle protein synthesis in young men. J Clin Endocrinol Metabol. 1999; 84: 2705–2711. [DOI] [PubMed] [Google Scholar]
- 40.Fuentes JJ, Pritchard MA, Estivill X. Genomic organization, alternative splicing, and expression patterns of the DSCR1 (Down syndrome candidate region 1) gene. Genomics. 1997; 44: 358–361. [DOI] [PubMed] [Google Scholar]
- 41.Musaro A, McCullagh KJ, Naya FJ, et al. IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature. 1999; 400: 581–585. [DOI] [PubMed] [Google Scholar]
- 42.Mitchell PO, Mills ST, Pavlath GK. Calcineurin differentially regulates maintenance and growth of phenotypically distinct muscles. Am J Physiol. 2002; 282: C984–992. [DOI] [PubMed] [Google Scholar]
- 43.Coux O, Tanaka K, Goldberg AL. Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem. 1996; 65: 801–847. [DOI] [PubMed] [Google Scholar]
- 44.Hochstrasser M. Ubiquitin-dependent protein degradation. Annu Rev Genet. 1996; 30: 405–439. [DOI] [PubMed] [Google Scholar]
- 45.Barrow RE, Dasu MRK, Ferrando AA, et al. Gene expression patterns in skeletal muscle of children treated with oxandrolone. Ann Surg (in press). [DOI] [PMC free article] [PubMed]
Footnotes
Presented at the 114th Annual Session of the Southern Surgical Association, December 1–4, 2002, Palm Beach, Florida.
Supported by NIH grants RO1-GM56687, P50-GM60338, T32-GM08256 and Shriners Hospital Grants 00-GAL-8770, 00-GAL-8660, and 00-GAL-8520.
Correspondence: Steven E. Wolf, MD, Shriners Burns Hospital, 815 Market, Galveston, TX 77550.
E-mail: swolf@utmb.edu
Accepted for publication December 2002.