Abstract
Objective
To compare the late clinical outcome and incidence of recurrent stenosis after carotid endarterectomy (CEA) with polytetrafluoroethylene (PTFE) versus Hemashield patching.
Summary Background Data
Several randomized trials have confirmed the advantages of patching over primary closure when performing CEA.
Methods
Two hundred CEAs (180 patients) were randomized into 100 with PTFE patching and 100 with Hemashield. All patients underwent postoperative color duplex ultrasounds at 1, 6, and 12 months, and every year thereafter. The mean follow-up was 26 months. Kaplan-Meier analysis was used to estimate the risk of re-stenosis, stroke, and stroke-free survival. A multivariate analysis of various risk factors was also done.
Results
Demographic and clinical characteristics were similar in both groups. The incidence of all ipsilateral strokes (early and late) was 8% (7% perioperative) for Hemashield versus 0% for PTFE patching. Both groups had similar mortality rates. The cumulative stroke-free rates at 6, 12, 24, and 36 months were 93%, 93%, 93%, and 89% for Hemashield versus 100%, 100%, 100%, and 100% for PTFE patching. The cumulative stroke-free survival rates at 6, 12, 24, and 36 months were 90%, 89%, 87%, and 79% for Hemashield versus 98%, 98%, 92%, and 92% for PTFE patching. Kaplan-Meier analysis also showed that freedom from 50% or greater re-stenosis at 6, 12, 24, and 36 months was 89%, 81%, 73%, and 66% for Hemashield versus 100%, 100%, 100%, and 92% for PTFE. Similarly, the freedom from 70% or greater re-stenosis at 6, 12, 24, and 36 months was 93%, 91%, 86%, and 78% for Hemashield versus 100%, 100%, 100%, and 100% for PTFE. Univariate and multivariate analyses of demographic and preoperative risk factors showed that only Hemashield was significantly associated with a higher incidence of 70% or greater recurrent stenosis.
Conclusions
PTFE patching was superior to Hemashield in lowering the incidence of postoperative ipsilateral strokes and late recurrent stenosis.
Carotid endarterectomy (CEA) has become one of the most common vascular procedures performed today. 1 Concerns regarding the morbidity and mortality of the procedure and lasting benefits compared to medical therapy alone have been discounted by prospective randomized trials that have validated the value of CEA in preventing cerebrovascular events in appropriately selected patients. 2–4 Initial procedures usually employ the concept of primary closure of the endarterectomized artery, but several authors have demonstrated reduction in the risk of perioperative stroke and late re-stenosis in arteries closed with patch angioplasty. 5–11 Available patch options include autologous vein or synthetic material. Two commonly used synthetic materials are polytetrafluoroethylene (PTFE; Gore-tex, W. L. Gore & Associates, Flagstaff, AZ) and collagen-impregnated knitted Dacron (Hemashield, Boston Scientific, Oakland, NJ). Multiple studies have shown the comparability of both PTFE 5,6,8,12 and Dacron 13–16 to autologous veins. With assumed effectiveness of all synthetic patches, Dacron has been advocated by some to avoid the prolonged hemostasis time associated with PTFE. 6,17 Dacron with collagen impregnation has been useful in large vessel replacement procedures. 18–20 In an earlier nonrandomized study, we demonstrated increased re-stenosis and perioperative stroke rates in CEA using Hemashield patching. 21 This was confirmed by the early results of this prospective randomized trial of PTFE versus Hemashield, which showed a significant increase in perioperative stroke and carotid thrombosis in the Hemashield group. 22 This present study compares the long-term clinical outcome and incidence of late re-stenosis after CEA with PTFE versus Hemashield patching.
METHODS
Patient Population
Two hundred CEAs (180 patients; 20 had bilateral CEAs) were randomized between July 1998 and March 2000 into 100 CEAs with PTFE patching and 100 CEAs with collagen-impregnated Dacron patching (Hemashield). Details of the randomization were described in our previously published study. 22 During the study period, the following patients were excluded from the trial: repeat CEAs (23 cases), CEAs with concomitant coronary artery bypass grafting (14 cases), and patients with an internal carotid artery diameter of less than 4 mm (none during this study). This study was approved by the Institutional Review Board of Charleston Area Medical Center/Robert C. Byrd Health Sciences Center of West Virginia University.
Before surgery, all patients underwent carotid color duplex ultrasound scanning and/or magnetic resonance angiography (MRA) to determine preoperative stenoses. Baseline blood cholesterol and triglyceride levels were also obtained. Various preoperative risk factors were determined, including smoking, hypertension, diabetes mellitus, coronary artery disease, and the preoperative use of antiplatelet therapy. The indications for CEA were categorized into hemispheric transient ischemic attack (TIA) symptoms, amaurosis fugax, hemispheric strokes, nonhemispheric TIAs, and asymptomatic carotid stenoses.
Operative Technique
All procedures were performed under general anesthesia with systemic heparin and routine shunting. No protamine was given at the end of the procedure. During surgery, the normal internal carotid artery distal to the lesion was measured in millimeters with calipers. Endarterectomies were extended proximally and distally beyond grossly diseased intima. PTFE cardiovascular patches (8 mm wide and 0.4 mm thick) were used and sutured with PTFE sutures (TT-9 needles, CV-6 sutures). Hemashield patches (8 mm wide) were sutured with 6-0 polyprolene sutures (Prolene, Ethicon, Somerville, NJ). Thrombin-soaked oxidized cellulose and digital pressure were applied to stop any bleeding points before closure in patients with PTFE patches. Completion postoperative duplex ultrasound scanning was performed on all patients. All patients were started on a regimen of aspirin (325 mg daily) within 24 hours of surgery.
Surveillance Protocol
All patients had clinical/neurologic examinations postoperatively and underwent immediate postoperative color duplex ultrasound scanning that was repeated at 30 days, 6 months, and every 6 months thereafter using an ATL HDI 5000 system (Advanced Technology Laboratory, Bellevue, WA). Reportable complications were determined in accordance with the Society of Vascular Surgery/American Association of Vascular Surgery Ad Hoc Committee Suggested Standards for Reports Dealing with Cerebrovascular Disease. 23 Peak systolic velocities of more than 140 cm per second on duplex examination with spectral broadening throughout systole and an increased diastolic frequency were consistent with hemodynamically significant stenoses (≥50% reduction). 24 Patients who had duplex findings that were consistent with severe stenoses of 70% or greater underwent MRA, conventional arteriogram, or carotid exploration (for patients with perioperative carotid thrombosis and neurologic deficits) to confirm their diagnosis.
Statistical Methods
The details of the statistical analysis were described in our previous study. 22 Morbidity rates and other noncontinuous variables were compared with either the chi-square test or Fisher exact test. Kaplan-Meier primary analysis was used to calculate the occurrence of late events (time to ≥50% re-stenosis or ≥70% re-stenosis or stroke). Multivariate analyses of various risk factors were performed as a means of determining independent predictors of perioperative stroke, carotid occlusion, and late re-stenosis. Possible risk factors were chosen based on an univariate analysis with one or two-tailed P of .1. Models were then refined by multiple runs (forward and backward), eliminating factors unlikely to be associated with outcome (i.e., high P value in these models).
RESULTS
As reported in our previous study, 22 there were no statistically significant differences in the demographic and clinical data for both groups. The 30-day perioperative complications were also reported earlier. 22 The mean follow-up was 25.5 months (range 1–40 months).
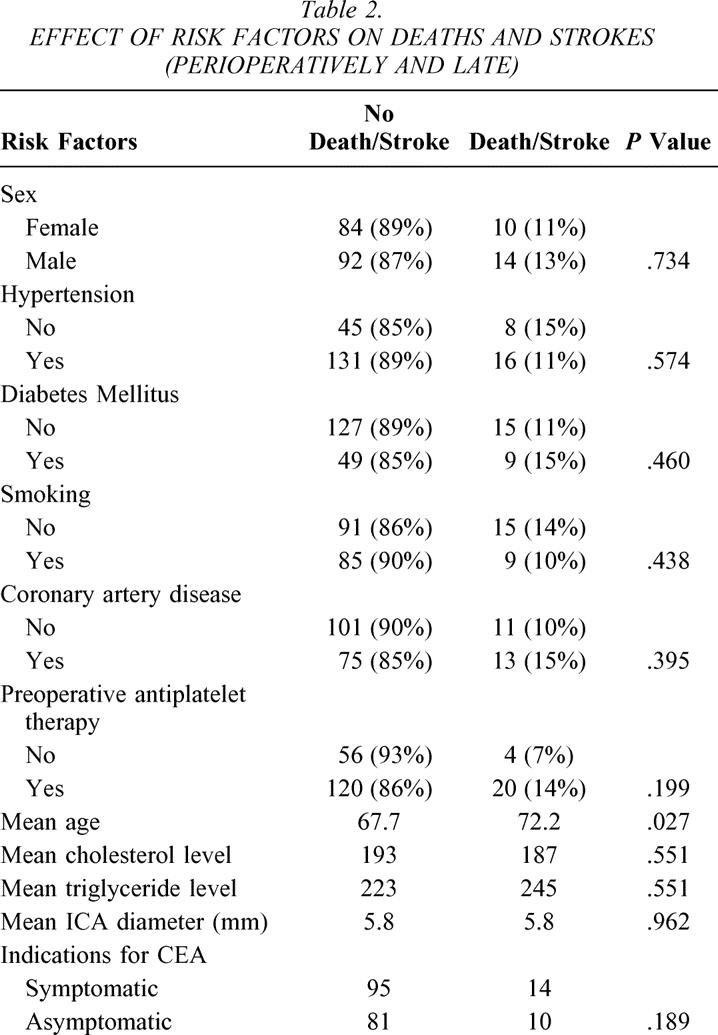
The incidence of all ipsilateral strokes (early and late) was 8% (7% perioperatively) for Hemashield patching versus 0% for PTFE patching (P = .012). Both groups had similar mortality rates. Table 1 summarizes the univariate analysis of various risk factors and the incidence of 70% or greater re-stenosis in the whole series. None of these risk factors was statistically significant. Table 2 summarizes the effect of various risk factors on all deaths and strokes (perioperative and late events). None of these factors was statistically significant except for age: the mean age for patients with stroke and death was 72.2 versus 67.7 for patients with no stroke or death (P = .027).
Table 1. UNIVARIATE ANALYSIS OF RISK FACTORS FOR ≥70% RE-STENOSIS IN THE WHOLE SERIES
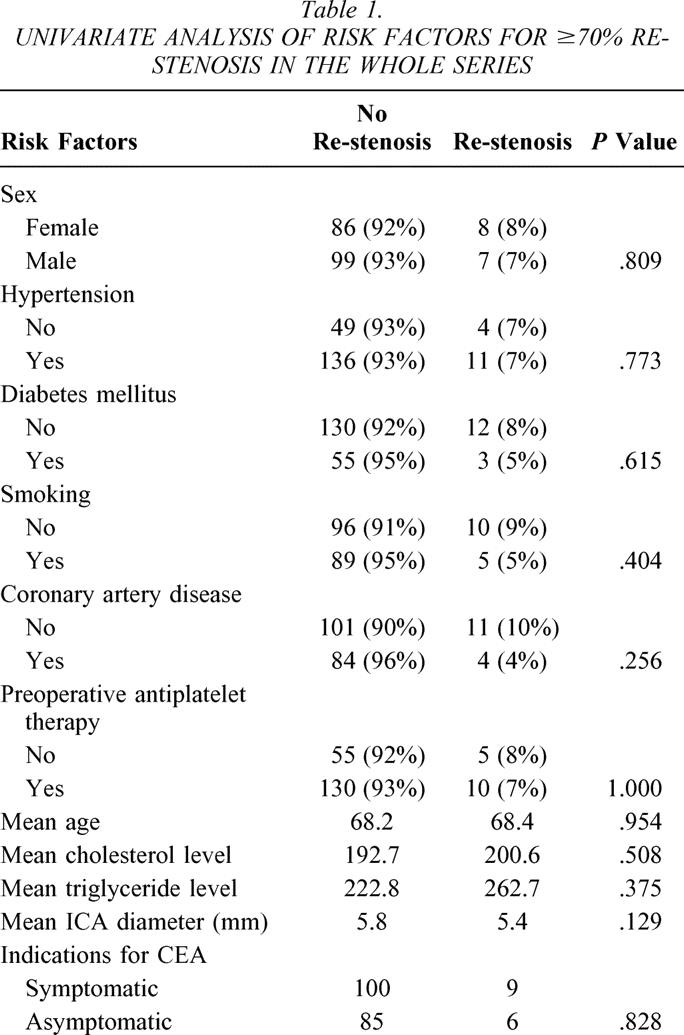
Table 2. EFFECT OF RISK FACTORS ON DEATHS AND STROKES (PERIOPERATIVELY AND LATE)
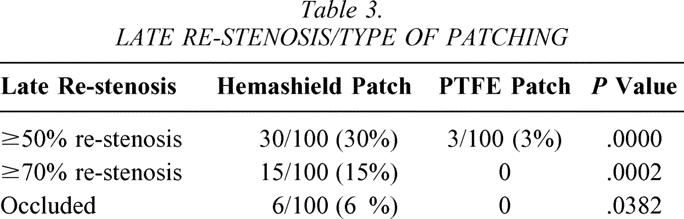
As noted in Table 3, 30% of patients with Hemashield patching had 50% or greater re-stenosis versus 3% for PTFE patching (P = .0000). Similarly, the incidence of 70% or greater re-stenosis was 15% for Hemashield patching versus 0% for PTFE (P = .0002). Carotid artery occlusion was noted in 6% of patients with Hemashield versus 0% for PTFE (P = .0382). All patients with 50% to 70% re-stenosis were asymptomatic except for one patient in the Hemashield patching group, who presented with TIA. Three patients with 70% to 99% re-stenosis were associated with TIAs, and one was associated with stroke. Five of six patients with carotid occlusion in the Hemashield group were noted perioperatively. 22
Table 3. LATE RE-STENOSIS/TYPE OF PATCHING
Univariate and multivariate analyses of demographic and preoperative risk factors showed that only the type of patching (Hemashield patching) was significantly associated with a higher incidence of recurrent stenosis of at least 70% (P = .0000).
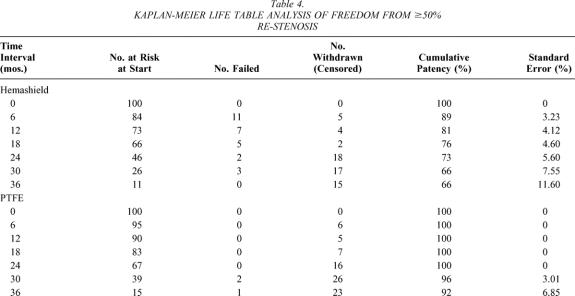
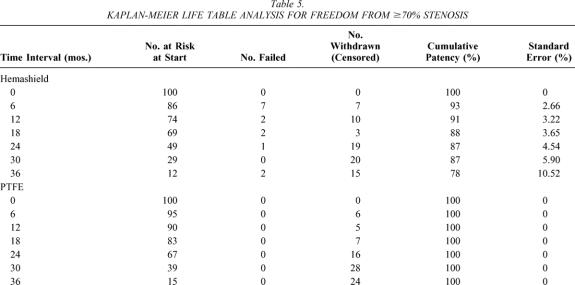
Kaplan-Meier analysis showed that freedom from 50% or greater re-stenosis at 6, 12, 24, and 36 months was 89%, 81%, 72%, and 66% for Hemashield patching versus 100%, 100%, 100%, and 92% for PTFE patching (P < .01, Table 4, Fig. 1). Similarly, the freedom from 70% or greater re-stenosis at 6, 12, 24, and 36 months was 93%, 91%, 86%, and 78% for Hemashield patching versus 100%, 100%, 100%, and 100% for PTFE patching (P < .01, Table 5, Fig. 2).
Table 4. KAPLAN-MEIER LIFE TABLE ANALYSIS OF FREEDOM FROM ≥50% RE-STENOSIS
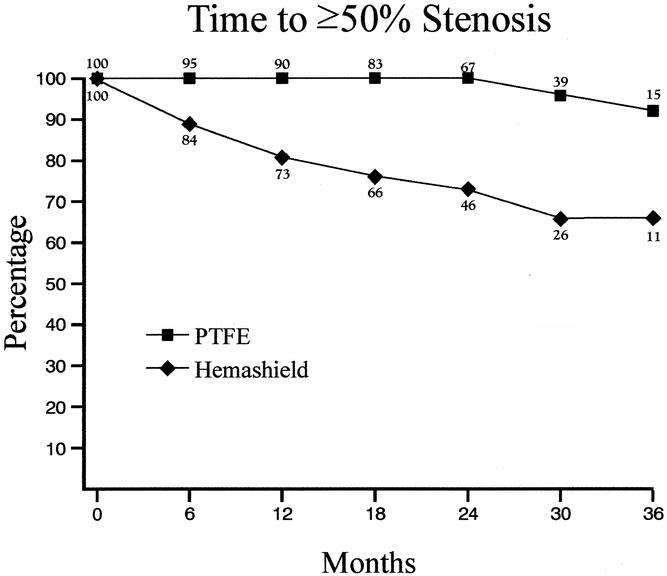
Figure 1. Kaplan-Meier life table analysis of freedom from 50% or greater restenosis.
Table 5. KAPLAN-MEIER LIFE TABLE ANALYSIS FOR FREEDOM FROM ≥70% STENOSIS
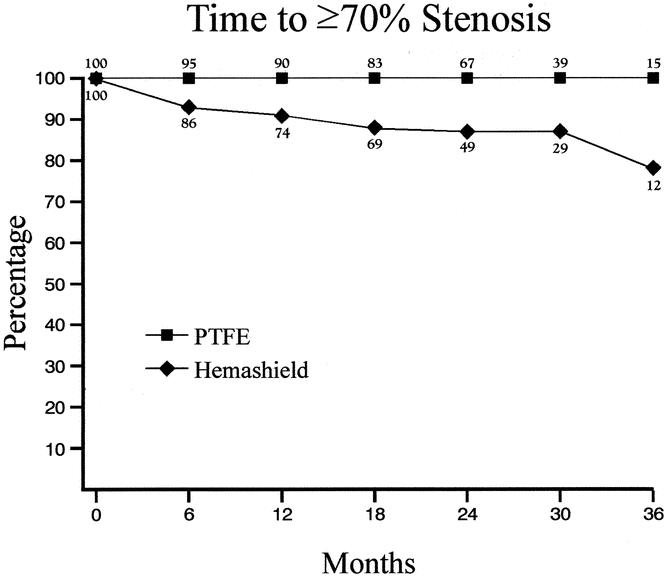
Figure 2. Kaplan-Meier life table analysis for freedom from 70% or greater stenosis.
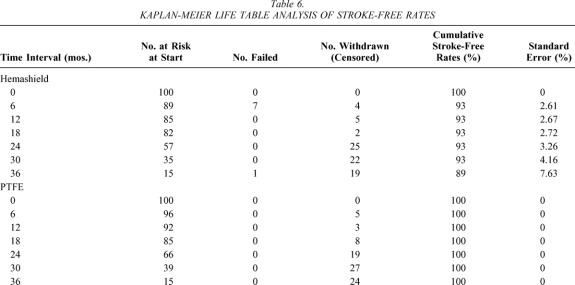
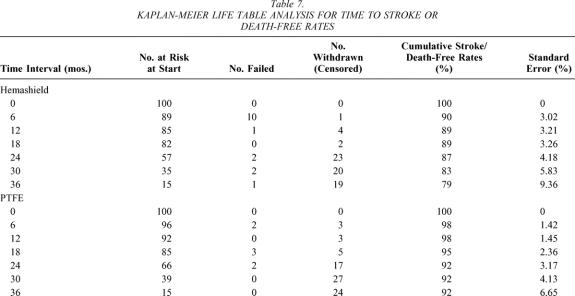
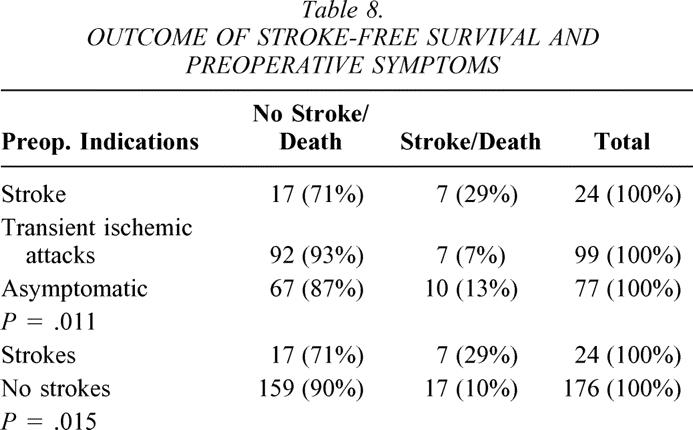
The cumulative stroke-free rates at 6, 12, 24, and 36 months were 93%, 93%, 93%, and 89% for Hemashield patching versus 100%, 100%, 100%, and 100% for PTFE patching (P < .01, Table 6, Fig. 3). The cumulative stroke-free survival rates at 6, 12, 24, and 36 months were 90%, 89%, 87%, and 79% for Hemashield patching versus 98%, 98%, 92%, and 92% for PTFE patching (P < .01, Table 7, Fig. 4). The outcome of stroke-free survival was highly associated with preoperative symptoms of stroke (vs. no symptoms or TIA), as noted in Table 8.
Table 6. KAPLAN-MEIER LIFE TABLE ANALYSIS OF STROKE-FREE RATES
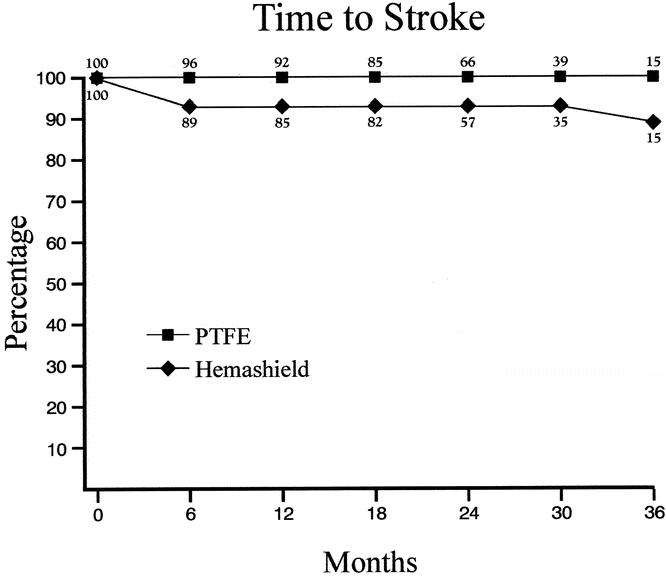
Figure 3. Kaplan-Meier life table analysis of stroke-free rates.
Table 7. KAPLAN-MEIER LIFE TABLE ANALYSIS FOR TIME TO STROKE OR DEATH-FREE RATES
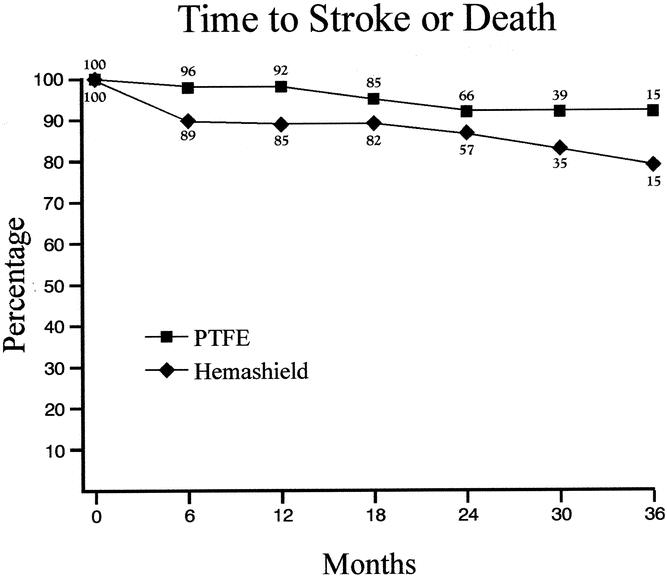
Figure 4. Kaplan-Meier life table analysis for time to stroke or death-free rates.
Table 8. OUTCOME OF STROKE-FREE SURVIVAL AND PREOPERATIVE SYMPTOMS
A multivariate analysis also showed that older patients (P = .0317) and preoperative indications of stroke (P = .0067) were the only factors associated with an increased incidence of subsequent stroke or death.
DISCUSSION
The practice of patch angioplasty with CEA has been shown by several authors to be more effective than primary closure with respect to results of perioperative stroke and re-stenosis. 5–13 The ideal patch has yet to be identified, with proponents for both autologous vein and synthetic grafts reaching no general consensus. Multiple studies have shown the comparability of both PTFE 5,6,8,13,25–28 and Dacron 13–16 to autologous vein patching. The advantages of synthetic patches include availability, decreased incidence of aneurysmal dilation or patch rupture, avoiding harvest site complications, and decreased operative times. Proponents of autologous tissue cite decreased infectious complications, less thrombogenic surface, and a decreased incidence of postoperative bleeding. Some researchers have demonstrated a prolonged hemostasis time, 6,8 as well as excessive bleeding from needle holes 17,29 in PTFE patches, while others have documented no significant bleeding with PTFE. 13,30
Reduction of blood loss with PTFE patching has been found to be associated with a needle/suture diameter ratio of 1:1. 30,31 In our patients, the use of PTFE suture (CV-6) and needles (TT-9) minimized the hemostasis time; however, the mean hemostasis time for the PTFE patches used in this study was still significantly higher than for Hemashield patching. Over the past 3 years, W. L. Gore & Associates, Inc., has introduced a new PTFE patch, Accuseal, that has a better hemostasis time than conventional PTFE patching.
Given this concern of excessive bleeding, the use of collagen-impregnated knitted Dacron patches has been advocated by some, without strong clinical evidence comparing the two synthetic patches. Before the early results of this study, 22 no prospective randomized trials were available evaluating these two patches.
In a previous prospective nonrandomized controlled study by our group, 21 a perioperative stroke rate of 4% was reported for patients closed using Hemashield patching, with a late re-stenosis rate of 21%. The re-stenosis rate was 22% for women and 14% for men.
The early results of this prospective randomized study reflected similar findings, with a higher incidence of perioperative strokes and carotid thrombosis in the Hemashield arm, suggesting that, in fact, these two synthetic materials are not equal. 22 The incidence of early re-stenosis was also significantly higher in the Hemashield group: 12% (this includes perioperative thrombosis) versus 2% for PTFE patching. This trend toward higher re-stenosis rates in the Hemashield group held true in the long-term follow-up.
Kaplan-Meier analysis showed that freedom from 70% or greater re-stenosis at 6, 12, 24, and 36 months was 93%, 91%, 86%, and 78% for Hemashield patching versus 100%, 100%, 100%, and 100% for PTFE patching (P < .01). Similarly, the cumulative stroke-free survival rates were superior for PTFE patching at 36 months (92% vs. 79%, P < .01).
The perioperative stroke rate in the Hemashield group was somewhat higher than we anticipated. However, in our previously published prospective controlled study of CEA with Hemashield patching (all performed by one surgeon, A.F.A.), the incidence of ipsilateral stroke was 5% (4% perioperatively), with a combined TIA and stroke rate of 12%. 21 The stroke rates in both studies were similar for the same surgeon (5.6% vs. 4%). Other studies comparing plain Dacron and vein patching have shown similar results. 14,15 Earlier studies have reported on the use of conventional Dacron patches in CEAs (i.e., non-collagen-impregnated Dacron patches), with postoperative stroke rates of approximately 3%. 9,10,14,15 In a retrospective study of vein versus Dacron patching, Goldman et al. revealed a re-stenosis rate of 8.4% with a mean follow-up of 13.7 months in the Dacron group. 14 Archie reported on the results of a nonrandomized series of 1,360 CEAs over 15 years, comparing three types of patches (saphenous vein, Dacron, and PTFE), and concluded that CEAs patched with saphenous veins were superior to CEAs patched with synthetic materials, particularly Dacron, for stroke and re-stenosis rates. 9 He also reported the results of a meta-analysis of three studies that contained both saphenous vein and Dacron patch reconstruction during CEA, and three other studies that contained both saphenous vein and PTFE patch reconstruction. The perioperative stroke rate for Dacron patching was 3% (598 CEA) versus 1% for vein patching (675 CEAs, P = .025). Similarly, the perioperative stroke rate for PTFE patching was 2.1% (331 CEAs) versus 0.3% for vein patching (363 CEAs, P = .056). Recently, Archie 32 analyzed 33 bilateral CEAs that were performed within 1 year in patients with paired vein and Dacron patch reconstruction, using a greater saphenous vein patch on one side and a knitted Dacron patch on the other side. Over a mean follow-up of 43 months, 10 (30%) Dacron-patched and 1 (3%) vein-patched CEA developed 25% or greater re-stenosis (P = .001), 7 (21%) Dacron-patched and no vein-patched CEA developed 50% or greater re-stenosis (P = .01), and 4 (12%) Dacron-patched and no vein-patched CEA developed 70% or greater re-stenosis (P = .11). He also reported that the cumulative 50% or greater re-stenosis rates for Dacron- and vein-patched CEAs were 16% and 0% at 2 years and 34% and 0% at 5 years, respectively (P = .003). The cumulative 70% or greater re-stenosis rates for Dacron- and vein-patched CEA were 8% and 0% at 2 years and 20% and 0% at 5 years, respectively (P = .02). He concluded that Dacron-patched CEAs have a significantly higher incidence of mild, moderate, and severe re-stenosis than do saphenous vein-patched CEAs, independent of systemic risk factors.
Ricco et al. 33 reported the results of using the ultra-thin collagen-impregnated knitted polyester patches (Hema-shield patch, Ultrathin, Intravascular) in 221 CEAs, with a 1.8% ipsilateral occlusion rate, a 1.4% ipsilateral stroke rate, and a 1.4% ipsilateral TIA rate. They also reported that the percentage of women free from 50% or greater re-stenosis was 82% at 3 years, compared with 90% for men for the same period (P = .035). Because of these results, they felt that the use of these patches should be restricted to men.
The data from this trial in both 30-day 22 and long-term follow-up raise the question of whether this Hemashield patch is thrombogenic in the endarterectomized carotid artery. Previously, collagen-impregnated Dacron has been praised for its handling characteristics, as well as hemostasis. Implantation of various grafts in dogs showed that medium-porosity knitted Dacron patches have better endothelial coverage, perigraft attachment, and thrombus-free surface than collagen-impregnated knitted Dacron patches. 34 Several reports on animal models have shown different characteristics of Dacron when used in the thoracic and abdominal aorta, 35 as well as in the carotid and femoral sites. 36 The local environment is different in the high-flow large-diameter aorta, compared to the endarterectomized carotid with exposed collagen and denuded adventitia. This local milieu may be responsible for the acute thrombo-genicity as well as the development of both early and late re-stenosis. Studies have shown increased platelet deposition on both PTFE and knitted Dacron, with a greater deposition on knitted Dacron. 37
In conclusion, PTFE patching is superior to Hemashield patching in lowering the incidence of postoperative ipsilateral strokes and late recurrent stenosis.
Discussion
Dr. Robert B. Smith, III (Atlanta, GA): Dr. AbuRahma and his colleagues in Charleston have an enviable record of conducting well-designed, randomized clinical trials and then following their patients carefully to obtain meaningful follow-up data. In this comparison of two commonly used carotid prosthetic patches, they have addressed an important, highly practical question: Which patch is better, Dacron or PTFE? Their data would tend to support the latter, but their manuscript leaves me still somewhat unconvinced, perhaps because I have used mostly Dacron for the past 20 years with very satisfactory results.
I have a number of questions related to technical issues that may have influenced their results. First, the 5% early occlusion rate in Dacron patch patients seems rather high and may reflect luxuriant platelet aggregation perioperatively. Did all patients receive preoperative aspirin or Plavix, and did you use intraoperative Dextran to reduce platelet adhesiveness further? Were the prosthetic patches applied to the entire length of the arteriotomy, or were some used only in the upper portion to assure adequate circumference of the internal carotid orifice? Did any patients undergo adjunctive steps to eliminate severe tortuosity of the vessel that might have predisposed to early postoperative kinking and occlusion? Finally, the marked difference in re-stenosis rate in Dacron versus PTFE patients is quite striking and suggests that thick myointimal hyperplasia is much more likely in the presence of Dacron, or perhaps its attached collagen coating. Are the authors aware that this observation has been made by other investigators, and what is the mechanism for this finding?
Dr. AbuRahma, as in any good study, you may have produced more questions than answers. I have every confidence, however, that you will return to West Virginia and undertake another well-designed trial that will further our knowledge in this area. We look forward to hearing more from you in the future.
Dr. R. Phillip Burns (Chattanooga, TN): I enjoyed this paper, and it does raise the dilemma that many of us have had in regard to utilization of these two materials in patching the carotid. I am curious as to what technique you used to stop the oozing and bleeding in your PTFE patches. I think a lot of us changed from PTFE years ago to the Hemashield patch because we had less problems with bleeding. So I would like to know, what are your secrets to stopping the bleeding after you release the clamps with the PTFE patch? I think it is a nicely done study.
Dr. Ward O. Griffen, Jr. (Frankfort, MI): I would like to just ask a methodological question. In your slides, I believe you showed that you used two different types of sutures in the two patches you studied. I wonder if it is the two different sutures rather than a difference in patches that made for the results.
Dr. Ali F. AbuRahma (Charleston, WV): I want to thank Dr. Smith for his nice comments, and I am honored to have him discuss this paper. In regard to his questions, it’s true that the 5% perioperative carotid occlusion rate was high in our series; in fact, it was surprisingly higher than we anticipated. However, I need to remind the audience that the past studies that reported a perioperative carotid occlusion rate that was lower than 5% used the conventional Dacron patch, not the collagen-impregnated Hemashield patch that we used in this study. Incidentally, this patch is also different from another collagen-impregnated Hemashield patch that is called the Ultrathin or Finess patch.
As we indicated in the manuscript, we are speculating that the presence of collagen in a very raw surface, such as the carotid endarterectomy surface, may be somewhat thrombogenic. However, our study was not designed to prove or disprove this speculation.
In regards to the length of the patch, the patch was not confined to the internal carotid artery; it was along the entire carotid endarterectomy incision.
In terms of using other antiplatelet agents, specifically dextran, none of our patients were treated with perioperative dextran therapy. However, all patients were given one aspirin daily. Perhaps using dextran may be a good option for those who prefer to use this patch.
In regards to any kinking that may explain the adverse outcome, there were no specific technical differences between patients who underwent PTFE and those who underwent Hemashield patching.
In regards to the second discussant and the issue of excessive bleeding using PTFE, I agree with the discussant. The conventional PTFE patches are known to be associated with an excessive hemostasis time; however, using thrombin and Gelfoam in our study minimized the hemostasis time to a mean of approximately 12 minutes. The hemostasis time can be shortened by using the new PTFE patch, Accuseal, that was introduced 3 years ago.
In regards to Dr. Griffen’s question regarding whether the difference in outcome may be related to the different suture materials used for PTFE versus the Hemashield patch, this study was not designed to answer that question. We simply used the sutures recommended by the PTFE Gore-tex company in suturing these patches; however, I doubt if this explains the difference in outcome.
I want to thank the Southern Surgical Association for the honor of presenting this paper. Thank you.
References
- 1.Cronenwett JL, Birkmeyer JD, eds. The Dartmouth Atlas of Vascular Health Care. Chicago: AHA Press, 2000:41–52. [PubMed]
- 2.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991; 325: 445–453. [DOI] [PubMed] [Google Scholar]
- 3.Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate to severe stenosis. N Engl J Med. 1998; 339: 1415–1425. [DOI] [PubMed] [Google Scholar]
- 4.Executive Committee of the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995; 273: 1421–1428. [PubMed] [Google Scholar]
- 5.AbuRahma AF, Khan JH, Robinson PA, et al. Prospective randomized trial of carotid endarterectomy with primary closure and patch angioplasty with saphenous vein, jugular vein, and polytetrafluoroethylene: Perioperative (30-day) results. J Vasc Surg. 1996; 24: 998–1007. [DOI] [PubMed] [Google Scholar]
- 6.AbuRahma AF, Robinson PA, Saiedy S, et al. Prospective randomized trial of carotid endarterectomy with primary closure and patch angioplasty with saphenous vein, jugular vein, and polytetrafluoroethylene: long-term follow-up. J Vasc Surg. 1998; 27: 222–234. [DOI] [PubMed] [Google Scholar]
- 7.Clagett GP, Patterson CB, Fisher DF Jr, et al. Vein patch versus primary closure for carotid endarterectomy: a randomized prospective study in a selected group of patients. J Vasc Surg. 1989; 9: 213–223. [DOI] [PubMed] [Google Scholar]
- 8.Allen PG, Jackson MR, O’Donnell SD, et al. Saphenous vein versus polytetrafluoroethylene carotid patch angioplasty. Am J Surg. 1997; 174: 155–157. [DOI] [PubMed] [Google Scholar]
- 9.Archie JP Jr. Patching with carotid endarterectomy: When to do it and what to use. Semin Vasc Surg. 1998; 11: 24–29. [PubMed] [Google Scholar]
- 10.Archie JP Jr. A fifteen-year experience with carotid endarterectomy after a formal operative protocol requiring highly frequent patch angioplasty. J Vasc Surg. 2000; 31: 724–735. [DOI] [PubMed] [Google Scholar]
- 11.Counsell CE, Salinas R, Naylor R, et al. A systematic review of the randomized trials of carotid patch angioplasty in carotid endarterectomy. Eur J Endovasc Surg. 1997; 13: 345–354. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Fajardo JA, Perez JL, Mateo AM. Saphenous vein patch versus polytetrafluoroethylene patch after carotid endarterectomy. J Cardiovasc Surg. 1994; 35: 523–528. [PubMed] [Google Scholar]
- 13.Rosenthal D, Archie JP Jr, Garcia-Rinaldi R, et al. Carotid patch angioplasty: Immediate and long-term results. J Vasc Surg. 1990; 12: 326–333. [PubMed] [Google Scholar]
- 14.Goldman KA, Su WT, Riles TS, et al. A comparative study of saphenous vein, internal jugular vein, and knitted Dacron patches for carotid endarterectomy. Ann Vasc Surg. 1995; 9: 171–179. [DOI] [PubMed] [Google Scholar]
- 15.Katz SG, Kohl RD. Does the choice of material influence early morbidity in patients undergoing carotid patch angioplasty? Surgery. 1996; 119: 297–301. [DOI] [PubMed] [Google Scholar]
- 16.Hayes PD, Allroggen H, Steel S, et al. Randomized trial of vein versus Dacron patching during carotid endarterectomy: Influence of patch type on postoperative embolization. J Vasc Surg. 2001; 33: 994–1000. [DOI] [PubMed] [Google Scholar]
- 17.McCready RA, Siderys H, Pittman JN, et al. Delayed postoperative bleeding from polytetrafluoroethylene carotid artery patches. J Vasc Surg. 1992; 15: 661–663. [DOI] [PubMed] [Google Scholar]
- 18.Reigel MM, Hollier LH, Pairolero PC, et al. Early experience with a new collagen-impregnated aortic graft. Am Surg. 1988; 54: 134–136. [PubMed] [Google Scholar]
- 19.Westaby S, Parry A, Giannopoulos N, et al. Replacement of the thoracic aorta with collagen-impregnated woven Dacron grafts: Early results. J Thorac Cardiovasc Surg. 1993; 106: 427–433. [PubMed] [Google Scholar]
- 20.Stegmann TH, Haverich A, Borst HG. Clinical experience with a new collagen-coated Dacron double-velour prosthesis. Thorac Cardiovasc Surg. 1986; 34: 54–56. [DOI] [PubMed] [Google Scholar]
- 21.AbuRahma AF, Robinson PA, Hannay RS, et al. Prospective controlled study of carotid endarterectomy with Hemashield patch: Is it thrombogenic? Vasc Surg. 2001; 35: 167–174. [DOI] [PubMed] [Google Scholar]
- 22.AbuRahma AF, Hannay RS, Khan JH, et al. Prospective randomized study of carotid endarterectomy with polytetrafluoroethylene versus collagen-impregnated Dacron (Hemashield) patching: Perioperative (30-day) results. J Vasc Surg. 2002; 35: 125–130. [DOI] [PubMed] [Google Scholar]
- 23.Baker JD, Rutherford RB, Bernstein EF, et al. Suggested standards for reports dealing with cerebrovascular disease. J Vasc Surg. 1988; 8: 721–729. [DOI] [PubMed] [Google Scholar]
- 24.AbuRahma AF, Richmond BK, Robinson PA, et al. Effect of contralateral severe stenosis or carotid occlusion on duplex criteria of ipsilateral stenoses: Comparative study of various duplex parameters. J Vasc Surg. 1995; 22: 751–762. [DOI] [PubMed] [Google Scholar]
- 25.Counsell C, Warlow C, Naylor R. Patches of different types for carotid patch angioplasty. Cochrane Database Syst Rev. 2000; 2: CD000071. [DOI] [PubMed] [Google Scholar]
- 26.Treiman RL, Foran RF, Wagner WH, et al. Does routine patch angioplasty after carotid endarterectomy lessen the risk of perioperative stroke? Ann Vasc Surg. 1992; 4: 317–319. [DOI] [PubMed] [Google Scholar]
- 27.Jacobowitz GR, Kalish JA, Lee AM, et al. Long-term follow-up of saphenous vein, internal jugular vein, and knitted Dacron patches for carotid artery endarterectomy. Ann Vasc Surg. 2001; 15: 281–287. [DOI] [PubMed] [Google Scholar]
- 28.Archie JP. Carotid endarterectomy outcome with vein or Dacron graft patch angioplasty and internal carotid artery shortening. J Vasc Surg. 1999; 29: 654–664. [DOI] [PubMed] [Google Scholar]
- 29.LeGrand DR, Linchan RL. The suitability of expanded PTFE for carotid patch angioplasty. Ann Vasc Surg. 1990; 4: 209–12. [DOI] [PubMed] [Google Scholar]
- 30.Rhodes VJ. Expanded polytetrafluoroethylene patch in carotid endarterectomy. J Vasc Surg. 1995; 22: 724–731. [DOI] [PubMed] [Google Scholar]
- 31.Miller CM, Sangiolo P, Jacobson JH II. Reduced anastomotic bleeding using new sutures with a needle-suture diameter of one. Surgery. 1987; 101: 156–160. [PubMed] [Google Scholar]
- 32.Archie JP Jr. Restenosis after carotid endarterectomy in patients with paired vein and Dacron patch reconstruction. Vasc Surg. 2001; 35: 419–427. [DOI] [PubMed] [Google Scholar]
- 33.Ricco JB, Bouin-Pineau MH, Demarque C, et al. The role of polyester patch angioplasty in carotid endarterectomy: a multicenter study. Ann Vasc Surg. 2000; 14: 324–33. [DOI] [PubMed] [Google Scholar]
- 34.Patel M, Arnell RE, Sauvage LR, et al. Experimental evaluation of ten clinically used arterial prostheses. Ann Vasc Surg. 1992; 6: 244–51. [DOI] [PubMed] [Google Scholar]
- 35.Hayashida N, Han MT, Wu MH, et al. Differential effect of the retropleural and retroperitoneal environment on healing of the inner wall of porous fabric prostheses in the thoracic and abdominal aorta of the same dog. Ann Vasc Surg. 1995; 9: 369–377. [DOI] [PubMed] [Google Scholar]
- 36.Durante KR, Wu HD, Sauvage LR, et al. Implant site: A determinant of completeness of arterial prosthesis healing in the dog and possibly in humans. Ann Vasc Surg. 1990; 4: 171–178. [DOI] [PubMed] [Google Scholar]
- 37.Wakefield TW, Shulkin BL, Fellows EP, et al. Platelet activity in human aortic grafts: a prospective, randomized midterm study of platelet adherence and release products in Dacron and polytetrafluoroethylene. J Vasc Surg. 1989; 9: 234–243. [DOI] [PubMed] [Google Scholar]
Footnotes
Presented at the 114th Annual Session of the Southern Surgical Association, December 1–4, 2002, Palm Beach, Florida.
Correspondence: Ali F. AbuRahma, MD, 3100 MacCorkle Ave., SE, Suite 603, Charleston, WV 25304.
E-mail: ali.aburahma@came.org
Accepted for publication December 2002.