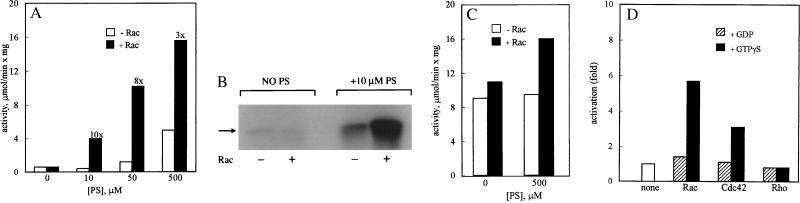
Figure 5.
Activation of MIHCK by Rac and Cdc42. (A) Phosphatidylserine-dependent activation of unphosphorylated MIHCK by Rac. MIHCK was incubated for 1 min at 20°C with synthetic peptide PC9 in the absence or presence of phosphatidylserine, at the indicated concentrations, and in the absence (open bars) or presence (closed bars) of GST–Rac–GTP[γS]. The fold-activation by Rac is shown at the top of the bars. (B) Autophosphorylation of MIHCK under identical conditions as in A. (C) Effect of Rac on the activity of phosphorylated MIHCK. MIHCK was fully autophosphorylated by incubation with ATP in the absence of phosphatidylserine and then assayed for 1 min at 20°C in the absence or presence of 500 μM phosphatidylserine and in the absence or presence of GST–Rac–GTP[γS], as indicated. (D) GTP[γS] dependence of activation of MIHCK by Rac and Cdc42. Unphosphorylated MIHCK was assayed in the presence of 500 mM phosphatidylserine and in the absence or presence of Rac, Cdc42, and Rho. All GTP-binding proteins were His-tagged at the N terminus and either in GTP[γS] or in GDP-bound form, as indicated in the figure. Activity was measured at 20°C for 1 min with Cdc42 and Rho and for 2 min with Rac. All values were normalized to the activity of MIHCK in the absence of p21s.