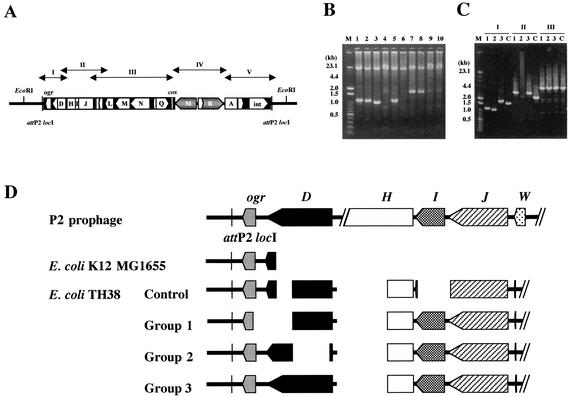
FIG. 6.
Variability of defective P2 prophage genomes in E. coli TH38 DNA. (A) Locations of target DNAs for PCR amplification. (B) Agarose gel electrophoresis of PCR products obtained from E. coli TH38 strains with primers I61 and I62. The PCR mixture (50 μl) comprised 25 pmol of each primer, 400 μM each deoxynucleotide triphosphate, 2.5 mM MgCl2, LA-PCR buffer (Mg2+ free), template DNA, and 2.5 U of LA Taq DNA polymerase. The reaction mixture was overlaid with mineral oil, and the reaction was carried out with a Perkin-Elmer Cetus thermal cycler. The initial template denaturation step consisted of 1 min at 94°C. The amplification profile (20 s at 98°C and 25 min at 68°C) was repeated for 30 cycles. Lanes 1 to 10 correspond to products from 10 E. coli TH38 colonies picked randomly. A mixture of λ HindIII digests and 100-bp DNA ladder markers (Toyobo) was used for molecular size calibration (lane M). The PCR products were subjected to 1% agarose gel electrophoresis and then stained with ethidium bromide. (C) Agarose gel electrophoresis of E. coli DNA fragments amplified by PCR with primers A and B (I), C and D (II), and E and F (III). The PCR mixture (50 μl) comprised 10 pmol of each primer, 200 μM each deoxynucleotide triphosphate, 2.5 mM MgCl2, LA-PCR buffer (Mg2+ free), template DNA, and 2.5 U of LA Taq DNA polymerase. The reaction mixture was overlaid with mineral oil, and the reaction was carried out with a Perkin-Elmer Cetus thermal cycler. The initial template denaturation step consisted of 1 min at 94°C. The amplification profile (30 s at 94°C, 1 min at 60°C, and 5 min at 72°C) was repeated for 25 cycles. Lanes C correspond to the products from the E. coli TH38 control strain, for which the complete nucleotide sequence of the 16-kb EcoRI region was determined in this study. Lanes 1 to 3 correspond to products with different molecular sizes from the E. coli TH38 strains analyzed in panel B. (D) Schematic diagram of the defective P2 prophage genome in various E. coli TH38 strains. Groups 1 to 3 correspond to lanes 1 to 3 in panel C.