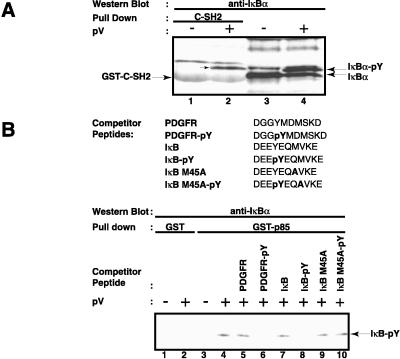
Figure 2.
Specificity of the interaction of tyrosine-phosphorylated IκB-α with the regulatory p85α subunit of PI3-kinase. (A) Interaction of tyrosine-phosphorylated IκB-α with the C-terminal SH2 domain of p85α. Two μg of GST-C-SH2 agarose conjugate (lanes 1 and 2) were incubated with whole-cell extracts from control (−) or 200 μM pV-treated (+) Jurkat T cells. The binding of IκB-α to the GST proteins was detected by Western blotting. The analysis of total cell extract by Western blotting for IκB-α is shown in lanes 3 and 4. The positions of IκB-α and its tyrosine-phosphorylated form, as well as that of the GST fusion protein, are indicated by arrows. (B) Specificity of interaction of tyrosine-phosphorylated IκB-α with p85α. Whole-cell extracts from control (−) and pV-treated (+) Jurkat T cells were incubated with 6 μM of the indicated peptides. Two μg of GST (lanes 1 and 2) or GST-p85α (lanes 3–10) agarose conjugates were added and the presence of IκB-α in the complexes was analyzed by Western blotting. The position of tyrosine-phosphorylated IκB-α is indicated.