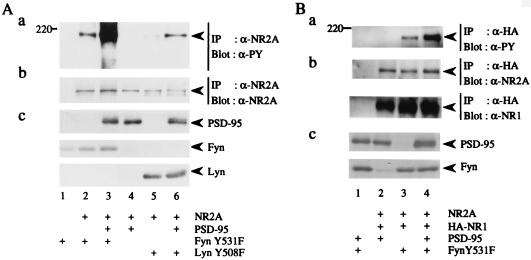
Figure 2.
Promotion of Fyn-mediated tyrosine phosphorylation of NR2A by PSD-95. (A) 293T cells were transfected with combinations of expression plasmids of NR2A, PSD-95, FynY531F, and LynY508F. Membrane fractions of the cells were lysed in TNE buffer. NR2A was immunoprecipitated (IP) and subjected to immunoblotting (Blot) with anti-PY antibody 4G10 (a). The filter used in a was reprobed with anti-NR2A. The doublet bands in the lane 5 and 6 may be caused by degradation of NR2A (b). The expression levels of PSD-95, FynY531F, and LynY508F were confirmed by immunoblotting (c). (B) 293T cells were transfected with combinations of expression plasmids of NR2A, HA-tagged NR1, PSD-95, and FynY531F. NR2A that formed complex with HA-NR1 was immunoprecipitated (IP) with anti-HA antibody and the HA immunoprecipitates were subjected to immunoblotting (Blot) with anti-PY antibody 4G10 (a). The filter used in a was reprobed with anti-NR2A (b Upper) and anti-NR1 (b Lower) to confirm the amounts of the proteins. The expression levels of PSD-95 and FynY531F were confirmed by immunoblotting (c). All experiments were performed more than four times. Positions and sizes (kDa) of standard protein markers are indicated. The positions of NR2A, HA-NR1 (120 kDa), PSD-95 (95 kDa), FynY531F (59 kDa), and LynY508F (56 kDa) are indicated by arrowheads.