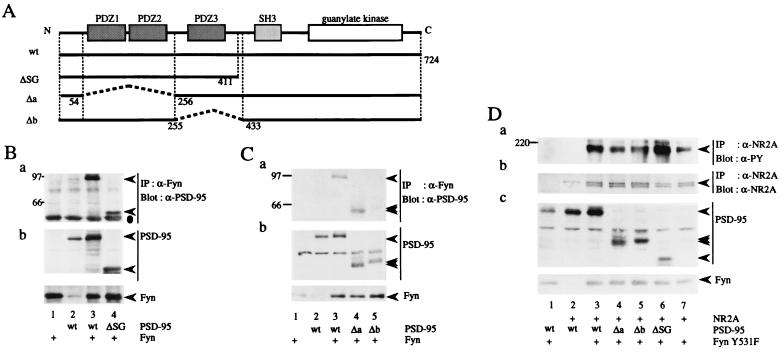
Figure 6.
Association of NR2A and Fyn with the distinct regions of PSD-95. (A) Schematic diagram of PSD-95 mutants. Residue numbers correspond to those in the amino acid sequence of PSD-95. The mutant ΔSG carried a deletion of 313 carboxy-terminal amino acids that included both the SH3 and guanylate kinase domains. The Δa mutant lacked 201 amino acids including the PDZ1 and PDZ2 domains. The Δb mutant carried a deletion of 177 amino acids including the PDZ3 domain. wt, wild-type. (B and C) 293T cells were transfected with combinations of expression plasmids of Fyn, PSD-95, or its mutants. Fyn immunoprecipitates (IP) were subjected to immunoblotting with anti-PSD-95 (a). The expression levels of PSD-95, Fyn, and their mutants were confirmed by immunoblotting (b). All experiments were performed more than three times. (D) 293T cells were transfected with combinations of expression plasmids of NR2A, FynY531F, PSD-95, and its mutants. NR2A immunoprecipitates were subjected to immunoblotting with anti-PY antibody 4G10 (a). The filter used in a was reprobed with anti-NR2A. The doublet bands may be caused by degradation of NR2A (b). The expression levels of FynY531F, PSD-95 and its mutants were confirmed by immunoblotting (c). The experiments were performed more than six times. Positions and sizes (kDa) of standard protein markers are indicated. The positions of NR2A, PSD-95, Fyn, and their mutants are indicated by arrowheads. The position of the heavy chain of anti-Fyn antibody is indicated by a dot in B.