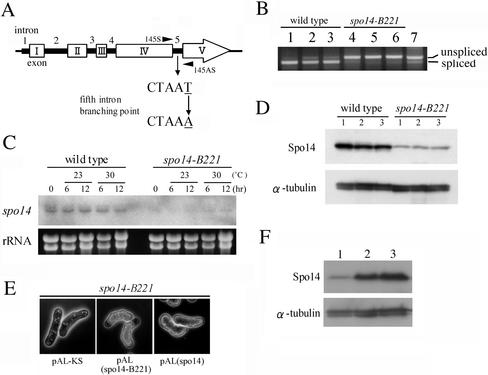
Figure 6.
Analysis of the spo14-B221 mutation. (A) Identification of the spo14-B221 mutation point. (B) RT-PCR analysis to assess splicing of the fifth intron. The PCR primers (145S and 145AS) are shown in Figure 6A. MKW5 (wild-type) and MK14 (spo14-B221) cells precultured in growth medium (MM+N) at 30°C were incubated in liquid sporulation medium (MM−N) at 23 or 30°C for 10 h. Strains: lanes 1–3, MKW5; lanes 4-6, MK14. Lanes 1 and 4, 0 h at 30°C; lanes 2 and 5, 10 h at 23°C, lanes 3 and 6, 10 h at 30°C. Lane 7, same as lane 1, but without DNase treatment. (C) Northern analysis of the spo14 mRNA in the spo14-B221 mutant. MKW5 and MK14 cells precultured in MM+N at 30°C were incubated in MM−N at 23 or 30°C. The equality of RNA loading was confirmed by staining gels with ethidium bromide (bottom). (D) Expression of Spo14 in the spo14-B221 mutant. MKW5 and MK14 cells precultured in MM+N at 30°C were incubated in MM−N at 23 or 30°C. Protein extracts were subjected to immunoblot analysis with the anti-Spo14 antibody, as well as anti–α-tubulin antibody as the loading control. Lane 1, 0 h at 30°C; lane 2, 10 h at 23°C; lane 3, 10h at 30°C. (E) Overexpression of spo14-B221 complemented the sporulation defect of the spo14-B221 mutant. Strain MK14L (h90 spo14-B221) carrying either pAL-KS, pAL(spo14-B221), or pAL(spo14) was incubated on SSA medium at 23°C for 5 d. (F) Expression of Spo14-B221 in the spo14-B221 mutant. MK14L cells transformed with either pAL-KS (lane 1) or pAL(spo14-B221) (lane 2) and MKW5 (lane 3) were precultured in MM+N at 30°C, and then cells were incubated in MM−N for 8 h. Immunoblot analysis was carried out as described in Figure 6D.