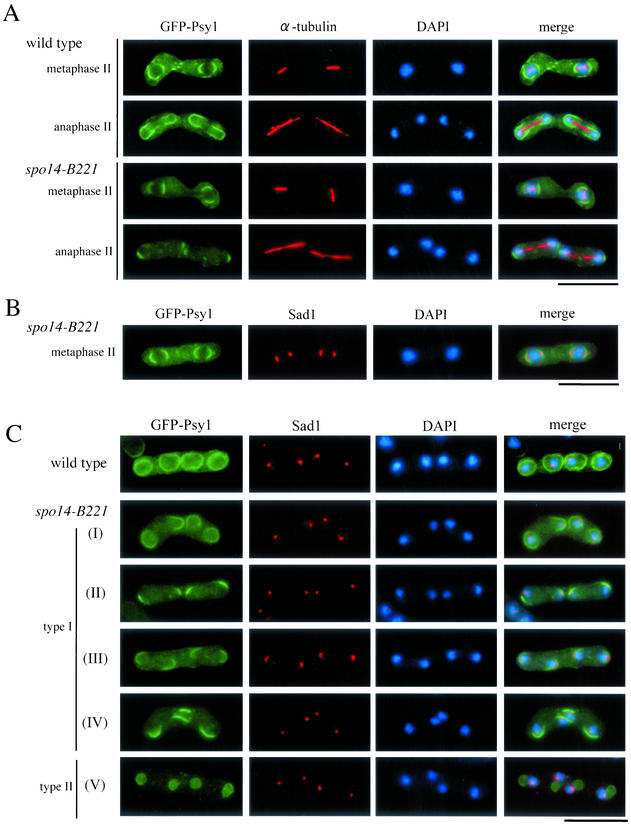
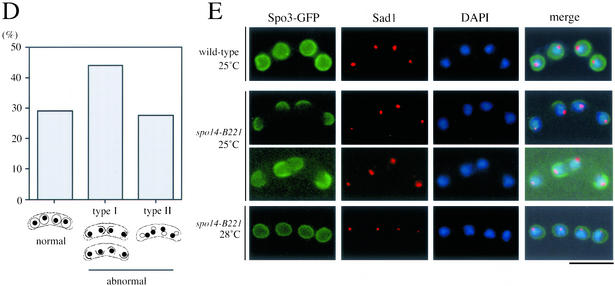
Figure 7.
Aberrant assembly of forespore membranes in spo14-B221. (A and B) Assembly of the forespore membrane during metaphase II and anaphase II. TN8 (wild-type) and MK14L (spo14-B221) transformed with pREP81(GFP-psy1) were cultured in SSL−N to induce meiosis at a semipermissive temperature (25°C) for 8 h. Fixed cells were doubly stained with the anti–α-tubulin (A) or anti-Sad1 antibody (B) and DAPI. Bars, 10 μm. (C) Classification of terminal phenotypes of the forespore membrane in spo14-B221 zygotes. Strain, culture conditions, and staining procedures are the same as described in Figure 7B. Type I, zygotes in which forespore membranes were formed at the normal site but did not extend; Type II, zygotes in which four aggregates of GFP-Psy1 were formed close to nuclei. (D) Relative frequency of the cell types of Figure 7C. Stained cells were categorized into three classes, normal, abnormal type-I and abnormal type-II. The values depicted show one representative result (N = 400) of three independent experiments. (E) Observation of forespore membrane using another forespore membrane marker, Spo3-GFP. MK14L cells were transformed with pAL(spo3-GFP) to visualize the forespore membrane by Spo3-GFP. Cells were incubated in SSL−N at either a permissive (28°C) or a semipermissive temperature (25°C). Fixed cells were doubly stained with anti-Sad1 antibody and DAPI. Bar, 10 μm.