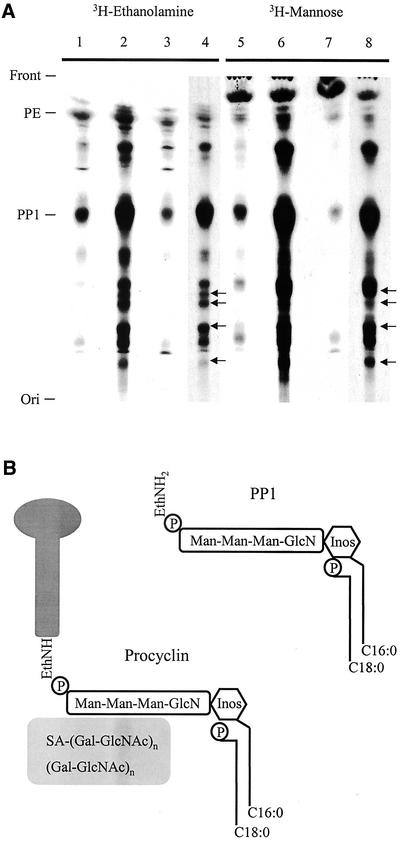
Figure 4.
Accumulation of lipids in Δgpi8. (A) Chloroform/methanol/water extracts of 3H-ethanolamine- and 3H-mannose–labeled procyclic forms were chromatographed and visualized by autoradiography. Δgpi8[GPI8] (lanes 1 and 5), Δgpi8 (lanes 2, 4, 6, and 8), and wild-type EATRO 795 (lanes 3 and 7). The positions of the front, origin, phosphatidylethanolamine (PE), and the major insect-stage T. brucei GPI-anchor precursor PP1 (Field et al., 1991b) are indicated at left. A number of novel lipid species were detected in Δgpi8 (indicated by arrows [←]). For clarity, the Δgpi8 extracts are shown after both 2-d (lanes 4 and 8) and 10-d (lanes 2 and 6) exposures. (B) Schematic illustrating the structural features of PP1 and the procyclin GPI anchor. Data are based on studies cited in the text. PP1, top right, contains a cannonical GPI-core glycan, together with an acyl-inositol headgroup (the major fatty acid here is palmitate, although other species are present) and a lyso-glycerolipid backbone (a single fatty acid, predominantly stearate, is attached to the glycerol sn-1 position). The procyclin GPI-anchor structure has the same core as PP1, including an identical fatty acid configuration. Additionally, the procyclin protein C terminus is in amide linkage to the ethanolamine, and there is a large heterogenous glycan of ∼15-kDa molecular weight, which contains sialic acid and is endo-β-galactosidase sensitive (indicating the presence of lactosamine repeat structures). Inositol is represented by a hexagon, the core glycan, and the side chain in the procyclin anchor by rounded rectangles, and the procyclin polypeptide by a lollipop. Diagram is not to scale.