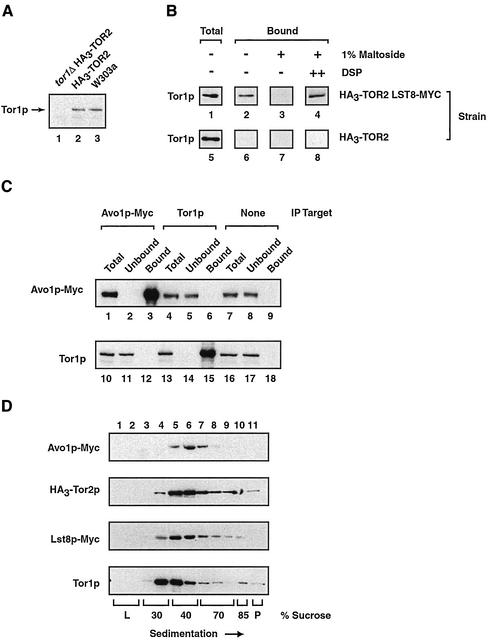
Figure 5.
Testing interactions between Tor1p and both Lst8p and Avo1p. (A) Demonstrating the specificity of an anti-Tor1p polyclonal antibody. Western blot analysis was performed on extracts prepared from the indicate strains. (B) Tor1p coprecipitates with Lst8p-Myc. Immunoprecipitation reactions were performed using anti-Myc antibodies and P100-AS fractions prepared from the indicated strains, followed by Western blot analysis to detect Tor1p. Where indicated, DSP was added at a final concentration of 1.8 mM before detergent solubilization. (C) Tor1p and Avo1p do not interact. Immunoprecipitation experiments were performed from extracts prepared from a strain that expressed Avo1p-Myc, by using either monoclonal anti-myc antibodies or polycolonal anti-Tor1p antibodies as indicated (IP target). Western blot analysis was then used to detect the presence of the indicated protein. Control beads indicates that Sepharose beads linked to protein G were used without a primary antibody. (D) Sedimentation profiles of Avo1p, Tor2p, Lst8p, and Tor1p. Clarified whole cell extracts were prepared from strain HA30TOR2 LST8-MYC or from strain AVO1-MYC and were subjected to ultracentrifugation of sucrose gradients. Fractions were then collected and analyzed by Western blotting to detect Avo1p-Myc, HA3-Tor2p, Lst8p-Myc, or Tor1p.