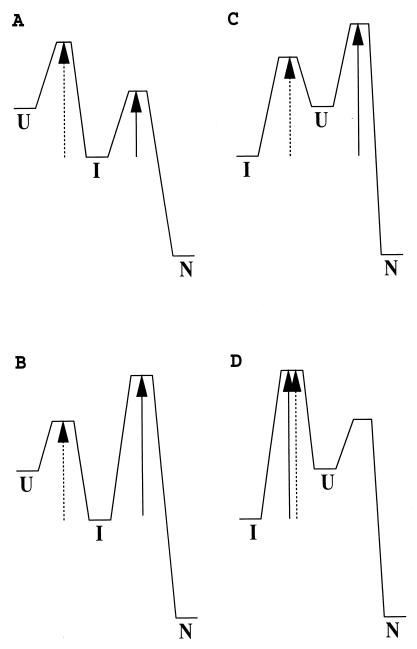
Figure 1.
Illustration of the kinetic difference between an on-pathway and an off-pathway reaction for an apparent three-state system. The effective kinetic barrier from I to N is indicated by the solid arrow and from I to U by the dashed arrow. (A and B) On-pathway reaction. (C and D) Off-pathway reaction. Only in A is kIN > kIU. This characteristic allows one to identify an on-pathway I. This is a sufficient but not a necessary condition. Even though kIN < kIU, I can still be on-pathway as in B. (C) The refolding I can be populated quickly, and a lag phase may be generated before the formation of N. In this case, U is not observable during the folding from I to N even though I must pass through U. Therefore, the appearance of a lag phase of I before formation of N does not necessarily indicate an on-pathway I (33). (D) Refolding may seem to be two-state, although a stable off-pathway I exists. Therefore, apparent two-state folding does not exclude the possibility that an equilibrium I may be observed. Similarly apparent two-state folding, as in A, can conceal the presence of an on-pathway I.