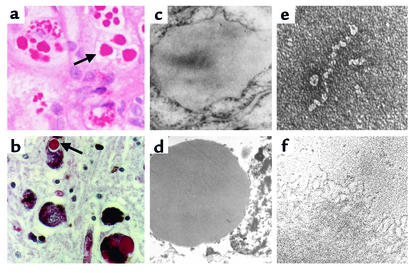
Figure 1.
Inclusion formation in the serpinopathies. Z α1-antitrypsin is retained within hepatocytes as intracellular inclusions, which are PAS-positive and diastase-resistant (a, arrow) and are associated with neonatal hepatitis and hepatocellular carcinoma. (c) Electron micrograph of a hepatocyte from the liver of a patient with Z α1-antitrypsin deficiency shows the accumulation of α1-antitrypsin within the rough endoplasmic reticulum. These inclusions are composed of chains of α1-antitrypsin polymers, shown here from the plasma of a Siiyama α1-antitrypsin homozygote (e) and from the liver of a Z α1-antitrypsin homozygote (f). Similar mutations in α1-antitrypsin deficiency and neuroserpin encephalopathy result in similar intracellular inclusions of α1-antitrypsin and neuroserpin. They are shown here in hepatocytes (a) and neurons (b) with PAS staining (arrow) and as endoplasmic aggregates of the abnormal proteins with electron microscopy (c, hepatocytes; d, neurons). Electron microscopy confirms that the abnormal neuroserpin forms beadlike polymers and entangled polymeric aggregates identical to those shown here with Z α1-antitrypsin (e and f, respectively). Magnification, left to right: ×200, ×20,000, ×220,000. Reproduced with permission from The New England Journal of Medicine (33).