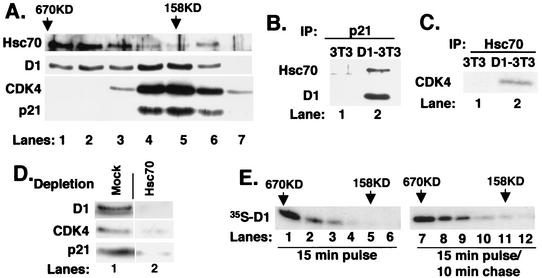
FIG. 3.
Cyclin D1 and Hsc70 are components of multiple complexes. (A) Affinity-purified cyclin D1 complexes were resolved by gel filtration. Ten percent of each fraction was subjected to immunoblot analysis with antibodies specific for Hsc70 (top panel), cyclin D1 (second panel), CDK4 (bottom panel), or p21Cip1 (bottom panel). (B and C) The fraction corresponding to lane 5 in panel A was precipitated with a p21-specific antiserum (B) or an Hsc70 monoclonal antibody (C). Associated proteins were monitored by immunoblot analysis with antibodies specific for the proteins indicated to the left of each panel. (D) The fraction corresponding to lane 5 of part A (158 kDa) was subjected to two rounds of immunodepletion with either normal rabbit antiserum (mock) or the Hsc70-specific monoclonal antibody. Codepletion of cyclin D1 (top panel), CDK4 (middle panel), and p21Cip1 (lower panel) was monitored by immunoblot analysis with the respective antibodies. (E) Asynchronous NIH 3T3 cells were pulse-labeled with [35S]methionine for 15 min (lanes 1 to 6) or pulse-labeled and subsequently chased for 10 min with excess unlabeled methionine for 10 min (lanes 7 to 12). Lysates prepared from these cells were resolved by gel filtration chromatography, and cyclin D1 was precipitated from fractions ranging from 670 kDa to 100 kDa (indicated at the top). Proteins were then analyzed by SDS-PAGE, and cyclin D1 was visualized by autoradiography.