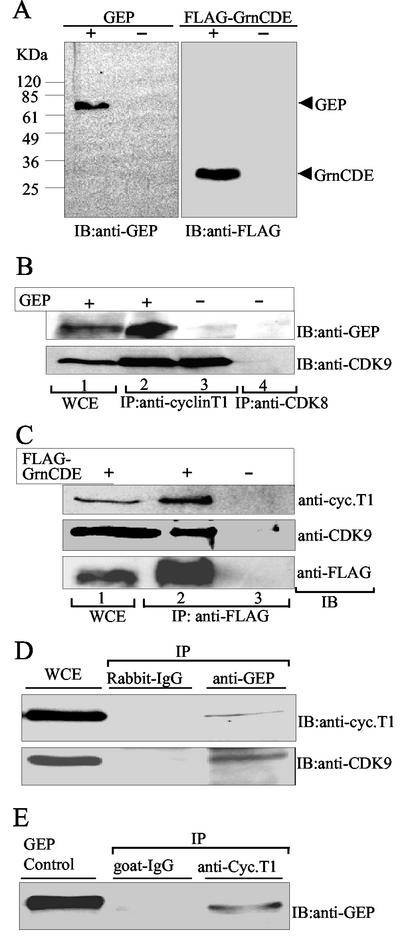
FIG. 4.
Granulin interacts with P-TEFb in vivo. (A) Immunoblot (IB) analysis of WCE from 293T cells overexpressing GEP or GrnCDE. GEP and FLAG-tagged GrnCDE were detected in extracts of transfected cells but not mock-transfected cells. (B) GEP coimmunoprecipitates with cyclin T1. Cyclin T1 complexes were immunoprecipitated (IP) from WCE prepared from 293T cells overexpressing GEP or mock transfected, as indicated (lanes 2 and 3). The immunoprecipitated complexes were separated in SDS-polyacrylamide gels, transferred to a membrane, and probed with anti-GEP or anti-CDK9 antibodies by immunoblotting (IB). Controls included unfractionated WCE (lane 1) and immunoprecipitation with anti-CDK8 (lane 4). (C) GrnCDE coimmunoprecipitates with CDK9 and cyclin T1. The immunoprecipitated complexes were probed with anti-FLAG, anti-CDK9. and anti-cyclin T1 antibodies, as indicated. Complexes containing GrnCDE, CDK9, and cyclin T1 were detected in extracts from 293T cells overexpressing FLAG-GrnCDE (lane 2) but not from cells transfected with empty vector (lane 3). (D) Endogenous cyclin T1 and GEP coimmunoprecipitate. Cyclin T1 complexes immunoprecipitated from WCE prepared from Daudi cells were probed with anti-GEP as described in for B. Controls included goat immunoglobulin G and purified GEP. (E) Endogenous GrnCDE coimmunoprecipitates with CDK9 and cyclin T1. Complexes were immunoprecipitated with anti-GEP and probed with anti-cyclin T1 and anti-CDK9 antibodies, as indicated. Rabbit immunoglobulin G was used as the control.