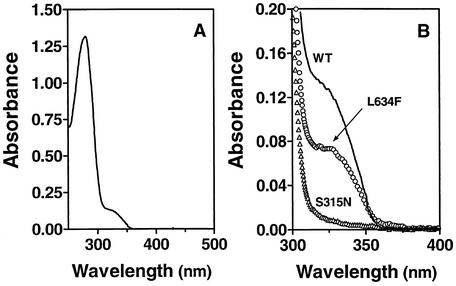
FIG. 3.
Isoniazid activation by wild-type and mutant KatG. The reactions were carried out for 3 h at room temperature with a sample of 1 ml of 50 mM phosphate, pH 7.0, containing 1 mM isoniazid, 1 mM NADH, and 0.5 μM wild-type KatG or a A110V, A139P, S315N, L619P, L634F, or D735A KatG mutant. KatG was then removed with Micron YM-10 filter units. One milliliter of sample containing 0.8 ml of filtrate and 0.2 ml of 78.5 μM InhA was incubated at room temperature for 20 min. The mixture was subjected to Sephadex G-25 column filtration twice, and the InhA-inhibitor complex was isolated. The spectra were normalized to a content of 10 μM InhA. (A) Absorption spectrum of the InhA-inhibitor complex with wild-type KatG for isoniazid activation. (B) Absorption spectrum of the same InhA-inhibitor complex derived from wild-type KatG (WT) activation of isoniazid is reproduced for the range of 300 to 400 nm. This spectrum is compared with those obtained under identical conditions with either the L634F or S315N KatG mutant in place of wild-type KatG. The final spectrum obtained for a negative control, in which wild-type KatG was used but no isoniazid was added, under otherwise identical conditions was identical to that shown for the S315N KatG mutant. For simplicity, spectra obtained with the other KatG mutants are not included. The corresponding yields of the inhibitor derived from isoniazid activation by all KatG species tested were calculated from the spectral data so obtained and are presented in Table 4.