Abstract
The activities of tigecycline (Wyeth Research) against extracellular and intracellular Legionella pneumophila and for the treatment of guinea pigs with L. pneumophila pneumonia were studied. The tigecycline MIC at which 50% of strains are inhibited for 101 different Legionella sp. strains was 4 μg/ml versus 0.125 and 0.25 μg/ml for azithromycin and erythromycin, respectively. Tigecycline was about as active as erythromycin (tested at 1 μg/ml) against the F889 strain of L. pneumophila grown in guinea pig alveolar macrophages and more active than erythromycin against the F2111 strain. Azithromycin (0.25 μg/ml) was more active than (F889) or as active as (F2111) tigecycline (1 μg/ml) in the macrophage model. When tigecycline was given (7.5 mg/kg of body weight subcutaneously once) to guinea pigs with L. pneumophila pneumonia, the mean peak serum and lung levels were 2.3 and 1.8 μg/ml (1.2 and 1.5 μg/g) at 1 and 2 h postinjection, respectively. The serum and lung areas under the concentration time curve from 0 to 24 h were 13.7 and 15.8 μg · h/ml, respectively. Thirteen of 16 guinea pigs with L. pneumophila pneumonia treated with tigecycline (7.5 mg/kg subcutaneously once daily for 5 days) survived for 7 days post-antimicrobial therapy, as did 11 of 12 guinea pigs treated with azithromycin (15 mg/kg intraperitoneally once daily for 2 days). None of 12 guinea pigs treated with saline survived. Tigecycline-treated guinea pigs had average end of therapy lung counts of 1 × 106 CFU/g (range, 2.5 × 104 to 3.2 × 106 CFU/g) versus <1 × 102 CFU/g for azithromycin (range, undetectable to 100 CFU/g). A second guinea pig study examined the ability of tigecycline to clear L. pneumophila from the lung after 5 to 9 days of therapy; bacterial concentrations 1 day posttherapy ranged from log10 4.2 to log10 5.5 CFU/g for four different dosing regimens. Tigecycline is about as effective as erythromycin against intracellular L. pneumophila, but tigecycline inactivation by the test media confounded the interpretation of susceptibility data. Tigecycline was effective at preventing death from pneumonia in an animal model of Legionnaires' disease, warranting human clinical trials of the drug for the disease.
Tigecycline (GAR-936; Wyeth Research) is a novel glycylcycline antimicrobial agent with previously unknown activity against Legionella bacteria, the causative agents of Legionnaires' disease. Tigecycline administered in an intravenous dosage of 100 mg to humans achieves maximal serum concentrations of ∼1 μg/ml and is well tolerated (G. Muralidharan, J. Getsy, P. Mayer, I. Paty, M. Micalizzi, J. Speth, B. Wester, and P. Mojaverian., Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1094, 1999). Prior studies with doxycycline and tetracycline have demonstrated that both drugs appear to be relatively inactive in vitro against L. pneumophila yet are active in an animal model of Legionnaires' disease and in the treatment of humans with Legionnaires' disease (2). Since the media used to grow L. pneumophila in vitro and in macrophages are known to inactivate tetracycline and doxycycline, it is important to assess the activity of tigecycline in animals as well as in vitro (2). This study examined the activity of tigecycline against a broad range of Legionella bacteria in vitro, against intracellular L. pneumophila, and in an animal model of L. pneumophila pneumonia.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
One hundred and one isolates of 37 different Legionella spp. were used to determine the in vitro activity of the study compounds. The species tested included one isolate each of the following species, except as noted: L. adelaidensis, L. anisa, L. birminghamiensis, L. bozemanii (3 isolates), L. brunensis, L. cherrii, L. cincinnatiensis, L. dumoffii (3 isolates), L. erythra, L. fairfieldensis, L. feeleii (3 isolates), L. geestiana, L. gormanii (2 isolates), L. gratiana, L. hackeliae, L. israelensis, L. jamestowniensis, L. jordanis, L. lansingensis, L. londiniensis, L. longbeachae (4 isolates), L. maceachernii, L. micdadei (4 isolates), L. moravica, L. nautarum, L. oakridgensis, L. parisiensis, L. pneumophila (50 isolates), L. quateirensis, L. quinlivanii, L. rubrilucens (3 isolates), L. santicrucis, L. shakespearei, L. spiritensis, L. steigerwaltii, L. tucsonensis, and L. wadsworthii. These bacteria represented almost all published Legionella spp. and included a large number of clinical isolates of L. pneumophila of all recognized serogroups. The vast majority (>95%) of these bacterial strains have been used in other studies of antimicrobial activity against Legionella spp. (5). Included in these strains were L. pneumophila strains F889 and F2111, which have been extensively studied in a cell model of L. pneumophila infection. Strain F889 has also been used extensively in a well-validated guinea pig model of L. pneumophila pneumonia (2-5, 11). Staphylococcus aureus ATCC 29213 was used as the control organism for susceptibility testing. To obtain inocula for susceptibility testing, legionellae were grown on MOPS (morpholinepropanesulfonic acid)-buffered charcoal yeast extract medium supplemented with 0.1% α-ketoglutarate that was made in our laboratory (BCYEα) (9) and nonlegionellae were grown on commercial tryptic soy agar containing 5% sheep blood (9). Incubation of all media was done at 35°C in humidified air for 24 to 48 h, depending on the organism and growth rate.
Antimicrobials.
Tigecycline standard powder was obtained from Wyeth Research. Tigecycline powder was dissolved in sterile water for injection, USP, and then in RPMI 1640 tissue culture medium, Mueller-Hinton broth, or buffered yeast broth, as appropriate. Tigecycline used in animal treatment and pharmacokinetic studies was dissolved in sterile water for injection, USP. Erythromycin “tissue culture-tested” standard powder was obtained from Sigma Chemicals and was first dissolved in 95% ethanol and then diluted in tissue culture medium or bacterial culture broth as described above. Azithromycin for injection (Pfizer) was used for antimicrobial susceptibility testing, macrophage studies, and the animal treatment study; this preparation contains no preservatives. Azithromycin used for the susceptibility studies was first reconstituted with sterile water for injection according to the manufacturer's recommendations, yielding a concentration of 100 mg/ml. The 100-mg/ml stock solution was aliquoted and frozen at −20°C. The frozen azithromycin stock solution was shown to maintain its expected biological activity against S. aureus throughout the period of the study. The tigecycline concentrations used for the pharmacokinetic and treatment studies ranged from 1 to 4 mg/ml and were prepared within 2 h of injection.
Antimicrobial susceptibility testing.
Broth microdilution susceptibility testing was performed using ACES [N-(2-acetamido)-2-aminoethanesulfonic acid]-buffered yeast extract broth supplemented with 0.1% α-ketoglutarate (BYEα) (Legionella) or Mueller-Hinton broth (non-Legionella bacteria), with a final volume of 100 μl and a final bacterial concentration of 5 × 105 CFU/ml (10). The Legionella and non-Legionella bacteria were incubated for 48 and 24 h, respectively, at 35°C. The BYEα broth was made in our laboratory.
Growth inhibition in alveolar macrophages.
Guinea pig pulmonary alveolar macrophages were harvested and purified as described previously (7), with one exception; the tissue culture medium used was RPMI 1640 with 15% fetal calf serum rather than M-199 with 20% fetal calf serum. The tissue culture medium was changed because the activity of tigecycline against S. aureus tested in M-199 with 20% fetal calf serum was more than four twofold dilutions greater than when Mueller-Hinton broth was used. The activities of tigecycline against S. aureus tested in RPMI with 10 or 20% fetal calf serum were one and two dilutions higher, respectively, than the drug's activity in Mueller-Hinton broth. The final concentration of macrophages was ∼105 cells per well. The incubation conditions for all macrophage studies were 5% CO2 in air at 37°C.
L. pneumophila strains F889 and F2111 grown overnight on BCYEα agar were used to infect the macrophages. Approximately 104 bacteria were added to each well. The bacteria were incubated with macrophages for 1 h in a shaking incubator and then for 1 day in stationary culture, as described previously (7). One set of replicate wells was washed (500 μl) three times with tissue culture medium and then sonicated at low energy to release intracellular bacteria, which were quantified using BCYEα agar. Antimicrobials were then added to the washed nonsonicated wells; several wells had no antimicrobial added and served as growth controls. The infected tissue cultures were incubated for 2 days, after which supernatant samples were taken for quantitative culture. The antimicrobials were then removed by washing the cultures, and the experiment continued for four more days, with daily quantification of L. pneumophila in the well supernatants. All experiments were carried out in duplicate or triplicate, and quantitative plating was done in duplicate. All wells were observed microscopically daily to detect macrophage infection and to roughly quantify the macrophages in the wells. To exclude tigecycline macrophage toxicity, control wells that contained macrophages, tissue culture medium, and antimicrobial agents but no bacteria were set up. The presence of macrophage toxicity was determined by microscopic observation of the cells. Prior studies had demonstrated no macrophage toxicity caused by erythromycin or azithromycin (8, 10). In this system, there is no extracellular growth of L. pneumophila, so all increases in supernatant bacterial concentrations are the result of intracellular growth.
Guinea pig pneumonia model.
Hartley strain male specific-pathogen-free guinea pigs (Charles River Laboratories, Wilmington, Mass.), ≈300 g in weight, were used for the pneumonia model, as previously described (4). The animals were observed for illness 1 week prior to infection; in the case of the animals used for the treatment study, temperatures and weights were obtained during the preinfection period. The guinea pigs were infected with L. pneumophila serogroup 1, strain F889, administered by the intratracheal route as previously described (4). The bacterial inocula administered were ≈5 × 106 CFU for both the pharmacokinetic and treatment studies.
Pharmacokinetic studies.
Tigecycline serum and lung specimens were obtained from guinea pigs with L. pneumophila pneumonia as described previously (3, 12). The drug was given in a single subcutaneous dose (7.5 mg/kg of body weight; 1.5 mg/ml in an ≈2-ml volume, with the injection volume dependent on individual animal weight) to the guinea pigs 1 day after infection; the mean guinea pig weight was 328 g. At timed intervals after drug injection, anesthetized (with ketamine, xylazine, and lidocaine) animals in groups of two or three were exsanguinated by the removal of heart blood under direct vision, followed by removal of the lungs. The heart blood was collected with a syringe and needle, transferred immediately to sterile glass tubes (Vacutainer; Becton-Dickinson, Rutherford, N.J.), allowed to clot at room temperature, and refrigerated (5°C) immediately. Within 1 h, the serum was separated from the cellular blood components by centrifugation at 5,000 × g and 5°C for 10 min and then stored frozen at −70°C until it was shipped to Wyeth Research on dry ice. Following removal, the lungs were rinsed in phosphate-buffered saline, drained on sterile cotton gauze, weighed, and ground in a known amount of sterile water; the final volume of the homogenate was measured to determine the lung weight per volume of final homogenate. Negative controls included guinea pig lung homogenate and serum that had been collected identically from normal guinea pigs given identical anesthesia but no antimicrobial. Additional multidose pharmacokinetic studies were performed using uninfected guinea pigs to support the interpretation of a bacterial clearance study of guinea pigs; the dosages used are given in Results below. In the multidose study, pharmacokinetic studies were performed on the last day of therapy.
Drug assay.
Tigecycline was assayed in serum and lung homogenates by Wyeth Research using a bioassay. Normal guinea pig lung homogenate or serum was used as the diluent in all assays and for the drug controls. Tigecycline levels in serum and lung homogenate samples were determined by a microbiological agar diffusion assay. Bacillus cereus ATCC 11778 spore suspension (Difco) was diluted 1:10 in sterile saline and incorporated at 1% (vol/vol) into nutrient broth with 1.1% agarose. Samples and standards were added (50 μl) to 8-mm-diameter wells cut in the agar. The plates were preincubated for 2 h at 4°C before being placed in a humidified 30°C incubator overnight. Unknown concentrations were determined based on zone size compared to known standards using a computerized system (Single Plate AutoAssay version 4.1.10; Giles Scientific, Santa Barbara, Calif.). The limit of detection for the assay was 0.06 μg/ml with a range of 0.06 to 2 μg/ml and a correlation coefficient of >0.98 for any given assay. Any samples containing drugs in amounts outside the linear range of the assay were reassayed after being diluted so that they fell within the assay range. Pharmacokinetic analysis was accomplished through noncompartmental modeling using WinNonlin version 3.2 (Pharsight Corp., Cary, N.C.). The lung homogenate and serum samples were analyzed as a single batch.
Animal treatment study.
The guinea pigs that survived the surgery required for administering the infectious inoculum were randomized into three treatment groups 1 day after infection. Starting on that day, treatment was given once daily (9:00 a.m.) for 2 to 5 days. One group of 16 animals received tigecycline (7.5 mg/kg subcutaneously in a 2-ml volume) for 5 days, and another 12 animals received azithromycin (15 mg/kg intraperitoneally [i.p.] in a 1-ml volume) for 2 days. The third group of 12 animals received saline solution once daily (1 ml i.p.) for 5 days. The tigecycline dose used was based on a pilot pharmacokinetic study conducted with two healthy guinea pigs and on expected human serum tigecycline concentrations (Muralidharan et al., Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother.). Animal weights and temperatures were taken periodically during the 12-day postinfection observation period; the measurements were taken immediately prior to drug administration on all treatment days. Necropsies and quantitative lung cultures were performed on all animals that died. All animals that survived for 12 days postinfection were killed with pentobarbital (150 mg/kg given i.p.). Necropsies, lung histopathologies, and quantitative lung cultures were performed on eight randomly selected animals each from the tigecycline and azithromycin treatment group survivors (4). The lower limit of detection of L. pneumophila in the lung was about 100 CFU/g. All animal studies performed at the University of Pennsylvania were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Bacterial clearance study.
Tigecycline clearance of L. pneumophila from the lungs of guinea pigs with L. pneumophila pneumonia was performed to determine the kinetics, magnitude, and reversibility of bacterial clearance. The guinea pigs were randomized into five different treatment groups 1 day after infection; four groups of 11 animals each were given tigecycline, and one group of 4 animals was given saline (1 ml i.p. daily for 2 days). The tigecycline treatment regimens were 7.5 mg/kg/day for 5 days (treatment group 1), 7.5 mg/kg/day for 9 days (treatment group 2), 15 mg/kg on day 1 and then 7.5 mg/kg daily on days 2 to 5 (treatment group 3), and 3.75 mg/kg twice daily for 5 days (treatment group 4). Starting on the day after infection, treatment was given once daily (9:00 a.m.) or twice daily (9:00 a.m. and 5:00 p.m.). All tigecycline was given in a 1-ml volume by the subcutaneous route in the flank; the injection site was rotated between sides for each injection. Animal weights and temperatures were taken periodically during the postinfection observation period; the measurements were taken immediately prior to drug administration on all treatment days. The animals were killed on a predetermined schedule, provided that they survived to their endpoint. Animals that became moribund or were in severe distress were killed before their respective endpoints. To avoid sampling bias, animals that died or were killed before their intended endpoints were not substituted for animals scheduled to be killed on that day. Necropsies and quantitative lung cultures were performed on all animals. The animals that survived to the predetermined study endpoint were killed with pentobarbital (150 mg/kg given i.p.). The lower limit of detection of L. pneumophila in the lung was about 100 CFU/g.
Statistical analysis.
All data analysis was performed with either Prism (version 3.02) or InStat (version 2.01) software (GraphPad, San Diego, Calif.). A two-tailed P value of ≤0.05 was predefined as significant. Body weight and temperature comparisons were analyzed using the methods specified for each result. Lungs with no detectable bacteria were arbitrarily assigned a value of 10 CFU/g to calculate and compare group mean values. WinNonlin version 3.2 (Pharsight Corp.) was used to calculate the pharmacokinetic parameters. The single serum sample containing no detectable drug was arbitrarily assigned a value of 0.0 μg/ml to allow its graphical representation and calculation of the pharmacokinetic parameters.
RESULTS
Broth dilution susceptibility.
The tigecycline MIC at which 50% of strains are inhibited, MIC at which 90% of strains are inhibited, and range for the Legionella spp. tested were 4, 8, and 0.5 to 8 μg/ml. The tigecycline MICs for L. pneumophila strains F889 and F2111 were 8 and 4 μg/ml, respectively. The respective erythromycin MICs were 0.25, 0.50, and 0.015 to 1 μg/ml. The azithromycin MICs were 0.125, 0.5, and 0.015 to 2 μg/ml. The highest tigecycline MICs were observed for L. pneumophila strains (geometric mean, 4.2 μg/ml, versus 1.7 μg/ml for non-L. pneumophila strains). In contrast erythromycin and azithromycin were about 1.5 times more active against L. pneumophila than the non-L. pneumophila strains tested. The BYEα test medium significantly inactivated tigecycline based on the tigecycline MICs for the control S. aureus strain tested in both Mueller-Hinton and BYEα media. Tigecycline was 6 to 45 times more active against S. aureus tested in Mueller-Hinton broth than in BYEα broth, depending on whether the comparison was made after 24 or 48 h of incubation, respectively. The respective values for azithromycin and erythromycin were 3.1 to 7.1 and 1.5 to 4. There was no correlation between the activities of tigecycline for particular strains and the activities of either erythromycin or azithromycin.
Antimicrobial inhibition of intracellular growth.
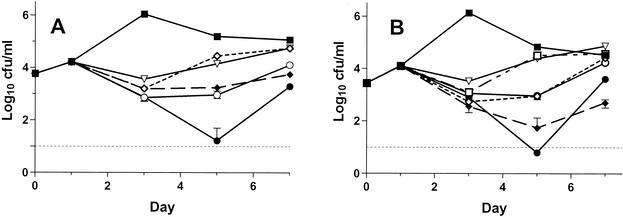
All three drugs tested significantly inhibited both L. pneumophila strains grown in guinea pig alveolar macrophages (Fig. 1). Tigecycline and erythromycin (0.25 and 1 μg/ml) had nearly identical and solely inhibitory activities against strain F889. Azithromycin was significantly more active against the same bacterial strain, with bactericidal activity and a long postantibiotic effect after extracellular drug removal, for the highest concentration (1 μg/ml) tested. Tigecycline (2 μg/ml) was about as active as azithromycin (0.25 μg/ml) against strain F889. Bacterial strain F2111 was more susceptible to the intracellular activity of tigecycline than was F889 in that a lower tigecycline concentration (1 μg/ml) was as active as azithromycin (0.25 μg/ml) against this strain and a higher tigecycline concentration (2 μg/ml) was bactericidal and demonstrated a prolonged postantibiotic effect. Tigecycline showed no evidence of toxicity for macrophages in drug-only control wells.
FIG. 1.
Growth of L. pneumophila serogroup 1 strains F889 (A) and F2111 (B) in guinea pig alveolar macrophages versus day of incubation after initiation of infection. The drugs were added on day 1 and removed by washing on day 3. All points represent the means of triplicate wells, each plated in duplicate; the error bars represent 95% confidence intervals, which unless shown were smaller than the height of the symbol representing the mean. ▪, saline control; □, tigecycline at 0.25 μg/ml; ⋄, tigecycline at 1 μg/ml; ♦, tigecycline at 2 μg/ml; ○, azithromycin at 0.25 μg/ml; •, azithromycin at 1 μg/ml; ▿, erythromycin at 1 μg/ml. The data for tigecycline at 0.25 μg/ml are not shown in panel A for greater clarity, as its effect was nearly identical to that of tigecycline at 1 μg/ml.
Pharmacokinetic studies.
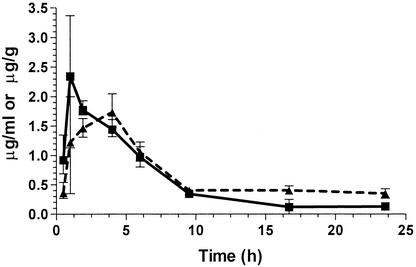
Tigecycline administration (7.5 mg/kg subcutaneously) to L. pneumophila-infected guinea pigs gave the highest mean measured serum drug concentrations of 2.3 and 1.8 μg/ml at 1 and 2.0 h, respectively (Fig. 2). The highest mean measured tigecycline concentrations in the lung were 1.5 and 1.7 μg/g, measured at 2 and 4 h, respectively. Only one serum sample analyzed had undetectable drug levels at 16.7 h postinjection; all lung samples tested had measurable drug levels detected. A two-phase exponential-decay model gave the best fit for the data and was used to calculate the half-life. Even so, the complex shapes of the curves prevented accurate estimation of elimination half-lives over the entire length of the study, resulting in two sets of half-life estimates. The serum terminal-phase (β-phase) half-lives of elimination were estimated to be 9.7 h (r = 0.81) over the period from 9.5 to 23.5 h postdose and 3.7 h (r = 0.98) over the period from 1 to 16.6 h postdose. The β-phase half-lives for the lung were estimated to be 61 (r = 0.86) and 2.6 (r = 0.99) h for the periods from 9.5 to 23.5 and from 4 to 9.5 h postdose, respectively. The area under the concentration curve from 0 to 24 h (AUC0-24) for serum was calculated to be 13.7 μg · h/ml, and that for the lung was 15.8 μg · h/ml.
FIG. 2.
Mean serum (solid line) and lung (dashed line) tigecycline concentrations in guinea pigs with L. pneumophila pneumonia. Animals were given a single 7.5-mg/kg dose administered by the subcutaneous route at time zero. Three animals were sampled at each time point except for the 24-h point, when two were sampled. One serum sample taken at 16.7 h contained no detectable drug. The error bars represent the ranges for the time points.
Tigecycline administration to normal guinea pigs resulted in higher concentrations in serum than in the lung, with shorter times to maximum concentration of the drug in serum (Table 1). The highest mean measured tigecycline concentrations in serum and lung were 6.9 μg/ml and 3.9 μg/g, respectively, for animals given 7.5 mg/kg for 10 days. Animals given the drug for 10 days had significantly higher serum and lung drug concentrations than those treated for 5 days. Uninfected animals had maximum serum and lung tigecycline concentrations that were 1.6 to 2.8 and 1.6 to 2.0 times, respectively, greater than the drug levels observed in infected animals. In addition, tigecycline concentrations in the lung peaked about 3 h later in infected than in uninfected animals. Twice-daily dosing had no apparent effect on the maximum concentration of drug in serum or the half-life, but it did increase the AUC by ∼30%. Administration of tigecycline to normal guinea pigs resulted in substantial weight loss; animals treated for 10 days with 7.5 mg/kg lost an average of 8.5% of their pretherapy body weight, while animals treated for 5 days lost an average of 5% of pretherapy weight regardless of dosage. Compared to the normal weight gain expected for young animals, the actual weight losses were 8 and 24%, respectively, for the 5- and 10-day therapies (Charles River Laboratories 2002 catalog [http://www.criver.com/02CAT/rm/other/hartleyGP.html]).
TABLE 1.
Serum and lung tigecycline concentrations and pharmacokinetic estimates for normal guinea pigs given the indicated doses
| Parameter | Valuea at dosage (mg/kg) of:
|
|||
|---|---|---|---|---|
| 7.5 for 5 days | 7.5 for 10 days | 7.5 BID for 5 days | 3.75 for 5 days | |
| Time postdose (h) | ||||
| 0.5 | 1.9/1.3 | 6.9/3.9 | 3.7/2.4 | 2.3/1.9 |
| 1 | 4.1/2.4 | 6.2/3.4 | 3.8/2.9 | 2.2/2.2 |
| 2 | 2.9/2.8 | 3.5/3.3 | 3.6/3.5 | 2.1/1.7 |
| 4 | 2.1/2.0 | 1.8/1.8 | 2.4/2.8 | 1.1/1.0 |
| 6 | 1.1/0.99 | 0.99/1.1 | 1.5/1.4 | 0.69/0.9 |
| 8 | 1/0.88 | 0.57/1.0 | 1.6/1.3 | 0.58/0.62 |
| 24 | 0.25/0.13 | 0.06/ND | 0.33/0.11 | 0.07/ND |
| AUC0-24 (μg · h/ml) | 28.7/22.7 | 24.9/21.3 | 38.4/30.3 | 15.8/13.1 |
| Half-life (h) | 8.2/6.0 | 4.6/3.5 | 7.4/4.7 | 5.4/3.9 |
Serum (micrograms per milliliter)/lung (micrograms per gram); n = 2 for each datum point. BID, twice a day; ND, not done.
Therapy in guinea pigs.
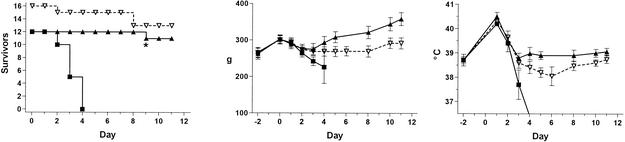
Thirteen of 16 guinea pigs treated with tigecycline survived for 12 days postinfection, as did 11 of 12 azithromycin-treated animals (P = 0.6 by Fisher's exact test). This was in contrast to 100% deaths among the 12 guinea pigs that received saline alone, a significant difference between the outcomes of the two treatment groups (P < 0.0001; chi-square test) (Fig. 3A). The one death in the azithromycin treatment group was the result of a toe injury which required euthanasia and not because of pneumonia. All of the other animals that died before day 12 had necropsy evidence of pneumonia.
FIG. 3.
Survival (left), body weights (middle), and rectal temperatures (right) of guinea pigs with L. pneumophila pneumonia versus day postinfection. Animals were treated on days 1 to 5 postinfection with saline or tigecycline (7.5 mg/kg) or for 2 days with azithromycin (15 mg/kg). The error bars represent 95% confidence intervals. ▿, tigecycline; ▴, azithromycin; ▪, saline control; *, animal euthanized because of injury unrelated to infection.
The animal weights and temperatures differed significantly between the two active-treatment groups from day 4 on, but not before (Fig. 3B and C). Saline-treated animals weighed significantly less than did animals in the other two groups on days 3 and 4, but not before (P < 0.01 by one-way analysis of variance). From day 4 on, the tigecycline-treated animals weighed significantly less than did the azithromycin-treated animals (P < 0.02 by unpaired t tests). Similarly, tigecycline-treated animals had lower body temperatures than did azithromycin-treated animals from day 4 on (P < 0.003 by unpaired t tests); the body temperatures of this treatment group on days 4 to 8 were significantly lower than the baseline normal body temperature of the group (P < 0.05 by unpaired t test).
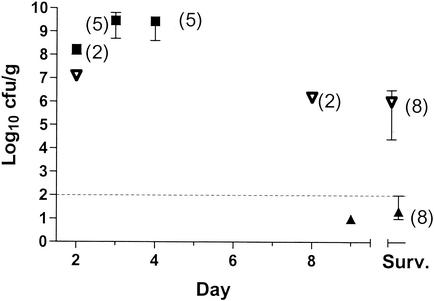
The lung culture and necropsy results for all saline-treated animals were diagnostic of fatal L. pneumophila pneumonia; the mean concentration of L. pneumophila was log10 9.4 CFU/g of lung, with a range of log10 8.0 to log10 9.8 CFU/g. The eight lungs examined from the tigecycline treatment group survivors contained an average L. pneumophila concentration of log10 6.0 CFU/g and a range of log10 4.4 to log10 6.5 CFU/g (Fig. 4). The tigecycline susceptibilities of persisting L. pneumophila bacteria were unchanged from that of the parent strain used to infect the animals. Only one of eight lungs examined from the azithromycin treatment group survivors contained detectable L. pneumophila (log10 2.0 CFU/ml).
FIG. 4.
L. pneumophila concentrations in the lung for animals that had necropsies. The surviving animals (Surv.) were killed on day 12 postinfection; thus, the data for days before day 12 are for animals that died. The number of animals represented by each data point is shown in parentheses; unlabeled points represent one animal. The error bars represent the range, which in some cases is smaller than the height of the symbol. ▿, tigecycline; ▴, azithromycin; ▪, saline control.
Histologic examination of lungs from the active treatment group survivors showed a significant difference in the amount of lung consolidation (n = 8 in each group; P = 0.03 by both unpaired t test and Mann-Whitney test). The median amount of lung consolidation in the tigecycline treatment group was 30%, with a 95% confidence interval of 17 to 53%; the values for the azithromycin treatment group were a median of 10% and a 95% confidence interval of 5 to 24%. The two tigecycline-treated animals that died on day 8 had 10 and 40% consolidation, both with acute focal neutrophilic pneumonia, which was different from the histologic pattern observed for the other survivors, which had a mixture of macrophages, neutrophils, and fibrosis.
Guinea pig lung bacterial clearance with tigecycline therapy.
Lungs from saline-treated animals contained an average of log10 9.3 CFU/g of L. pneumophila 2 days after infection (Table 2). Three tigecycline-treated animals died of pneumonia on the same day, two in group 4 and one in group 1; the lungs from these animals contained about 300-fold fewer bacteria than did those of the saline-treated animals. Lungs from animals treated for 5 days with tigecycline contained log10 4.1 to log10 4.8 CFU/g 1 day after the end of therapy. Bacterial lung counts from these animal treatment groups increased by about 1 log10 CFU/g over a 2-day period and remained relatively constant over the next 3 days. No significant differences were apparent in bacterial clearance among the three different 5-day treatment groups. There was no significant difference in bacterial clearance for animals given 9 rather than 5 days of therapy. Tigecycline treatment resulted in hypothermia and weight loss for all animal groups, but these adverse effects were reversible after the cessation of therapy (data not shown). Treatment with tigecycline for >5 days was fatal for a total of three animals on days 8 and 10 postinfection; these animals experienced severe weight loss and anorexia but no diarrhea. These deaths and the severe weight loss in this group prompted cessation of therapy, which had been planned for 10 days rather than 9 days. Necropsies of the long-duration therapy group showed gross findings that were not different from those for animals in the other tigecycline treatment groups.
TABLE 2.
L. pneumophila lung clearance from infected guinea pigs treated with tigecycline
| Treatment dosage (mg/kg)a | Log10 CFU/g on postinfection dayb:
|
||||
|---|---|---|---|---|---|
| 2 | 6 | 8 | 10 | 12 | |
| 7.5 (5 days) | 6.7 | 4.8 (3.3-5.1; 4)b | 6.2 (5.6-6.6; 4) | 5.7 (4.8-6.0; 2) | |
| 7.5 (9 days) | 4.8 | 5.5 (2.3-5.4; 6) | 4.9 (4.4-5.9; 4) | ||
| 15 (day 1); 7.5 (4 days) | 4.8 (3.5-5.4; 4) | 6.6 (5.1-7.0; 3) | 4.8 (4.4-5.1; 4) | ||
| 3.75 BID (5 days) | 6.9 (6.4-7.2; 2) | 4.2 (3.3-4.5; 3) | 5.7 (5.6-5.9; 3) | 5.9 (5.7-6.1; 3) | |
| Saline | 9.3 (9.0-9.6; 4) | ||||
BID, given twice daily.
Mean (range; n); for cases where n = 1, no range or n is given.
DISCUSSION
These results show that tigecycline is active against L. pneumophila in vitro and against the bacterium when it is growing in cells. In addition, tigecycline prevented death from pneumonia in the guinea pig model of L. pneumophila pneumonia. The in vitro and intracellular activities of tigecycline were difficult to determine with accuracy because the extracellular drug was partially inactivated by the test media. While tigecycline prevented deaths in the animal treatment model, it was clearly ineffective at clearing the bacterium from the lungs.
It is known that tetracycline and doxycycline are inactivated by iron, a major component of the BYEα growth medium used for L. pneumophila (2). In addition, the iron, calcium, and magnesium present in tissue culture medium can inactivate this class of drugs. These studies demonstrate that BYEα broth and RPMI 1640 medium partially inactivate the antimicrobial activity of tigecycline.
The greater activity of tigecycline against Legionella spp. other than L. pneumophila is unusual, as erythromycin and azithromycin are both more active against L. pneumophila than against the non-L. pneumophila Legionella spp. Some other antimicrobial agents, such as the fluoroquinolones and clarithromycin, are equally active against both types of Legionella bacteria. The significance of this finding is unknown, as is its mechanism. Since the majority of cases of Legionnaires' disease are due to L. pneumophila, the best measure of the activity of tigecycline for Legionella spp. is its activity against L. pneumophila.
Tigecycline was about as active as erythromycin in the macrophage infection model. This is despite partial inactivation of the extracellular drug by the tissue culture test medium. Knowledge of the intracellular concentration of tigecycline, as well as a determination of the subcellular distribution of the drug, would help to explain the discrepancy between the extracellular tigecycline MIC and its intracellular activity. Since only biologically active intracellular drug distributed in the lysosomal compartment is active against intracellular L. pneumophila in this model system, and most likely in humans, it is clear that sufficient tigecycline is present inside alveolar macrophages (2).
The significance of the greater activity of tigecycline for strain F2111 in macrophages is unknown. The extracellular tigecycline MICs for strains F2111 and F889 were 4 and 8 μg/ml, respectively. It is possible that this twofold difference in the MIC could have a significant impact on intracellular activity, although such a twofold MIC difference could also be analytically insignificant.
Tigecycline was only inhibitory against intracellular L. pneumophila, except when tested against strain F2111, and only at a high concentration (2 μg/ml). This places the drug in a group of other compounds that have only inhibitory activity against the bacterium, such as erythromycin and clarithromycin, which are still effective for the treatment of Legionnaires' disease.
Given the relatively good intracellular activity of tigecycline against strain F889, the performance of the drug in the animal model was unexpected. Several possible explanations exist for these findings. One possibility is that tigecycline administration was deleterious to the guinea pigs and that this masked the clinical signs of improving infection. Indeed, it is possible that tigecycline administration exacerbated the infection. Hypothermia and weight loss, usually signs of unresponsive L. pneumophila pneumonia, were found to be due rather to tigecycline administration, based on studies of uninfected animals and reversal of both hypothermia and weight loss after drug cessation in infected guinea pigs. The severity of guinea pig illness caused by prolonged tigecycline administration was shown by several late deaths in the prolonged-treatment group, unaccompanied by bacterial relapse. It is important to note that many antimicrobial agents can be fatal to guinea pigs but not to humans, including penicillin G and other β-lactam drugs, erythromycin, and rifampin (3), and that toxicity of the type that we observed in tigecycline-treated guinea pigs has not been observed in humans (R. Testa, personal communication). Another possibility for the less-than-expected tigecycline activity in the animal model is that tigecycline failed to reach extracellular concentrations sufficient to result in intracellular concentrations similar to those studied in the macrophage experiments. Extracellular concentrations as low as 0.25 μg/ml effectively prevented intracellular growth in the macrophage experiments, a level achieved in the sera of guinea pigs for 9 to 16 h after drug administration. However, drug binding or cationic complexing by guinea pig serum could have been substantially greater than was the case for the macrophage culture medium.
The most telling data in the animal treatment study are the persistence of L. pneumophila in the lungs at the end of tigecycline therapy. Bacterial persistence is observed for some other drugs, in particular erythromycin (2). However, the levels of L. pneumophila observed in the lungs of animals treated with erythromycin are ∼10- to 100-fold lower than those observed for the tigecycline-treated animals (4, 13). Animals treated with levofloxacin contain an average of 103 CFU of residual L. pneumophila per g of lung at the end of a study, or about 100 to 1,000-fold less than the 105 to 106 CFU/g observed for tigecycline (14). In a study of doxycycline therapy (8 mg/kg/day given i.p. once daily for 5 days) in the same animal model and using the same F889 bacterial strain (doxycycline MIC, 1 μg/ml), the residual bacterial concentrations were ∼104 CFU/g (4). The concentrations of doxycycline in the serum and lung in two animals in that study 1 h after injection were 0.4 and 0.7 μg/ml and 0.1 and 0.2 μg/g, respectively, significantly lower than those observed for tigecycline. It is usual for the residual bacterial count to be undetectable in the majority of azithromycin-treated animals, as was observed in this study. It is important to know that azithromycin is one of the most active drugs known in this animal model and that almost all other drugs have performances inferior to it in terms of residual bacterial clearance and lung inflammation (6, 14-17). Thus, the residual bacterial concentrations in the lung observed in this study are significantly greater than those usually observed for comparator drugs, even for one of the same drug class. These findings suggest that tigecycline, at the dose given, is weakly active in this animal model.
The histologic results showing that tigecycline-treated animals had greater amounts of lung consolidation than azithromycin-treated animals is a finding observed for other drug treatments in comparison with azithromycin (14, 15). In a recent study, erythromycin-treated animals had ∼22% consolidation in survivors (13), comparable to that observed with tigecycline treatment, and in all studies the amount of consolidation observed with azithromycin treatment is usually ∼10%. Saline-treated animals generally have ∼75% lung consolidation at the time of death (13). Thus, the amount of lung consolidation observed for the tigecycline-treated survivors is consistent with effective early therapy that limited bacterial multiplication in the lungs and prevented extensive lung damage.
Tigecycline levels in uninfected animals given a multidose regimen were greater than those observed in single-dose infected guinea pigs. It is known that some antibiotics are cleared more rapidly or differently in infected animals, presumably because of higher metabolic rates and changes in water composition in the inflamed lung (19). Another possible explanation is that multidosing resulted in higher tigecycline concentrations. For the first case, we may have overestimated drug exposure in the bacterial lung clearance study, but possibly not in the second case.
The lung clearance studies showed that tigecycline therapy quickly cleared several log units of L. pneumophila from the lung. However, after drug treatment ended, bacteria that had been inhibited but not killed regrew within the lung, similar to the regrowth of intracellular bacteria in the macrophage study. The residual bacterial lung concentrations were independent of the tigecycline dosage given, despite up to twofold differences in the maximum concentrations in serum. The AUC0-24 values for the different dosages studied were similar to one another, suggesting that this parameter is most important in determining bacterial clearance from the lung with tigecycline treatment. The reason for the relative constancy of the residual bacterial lung concentration, even with prolonged therapy, is unclear. Development of drug resistance by the lung bacteria was excluded by in vitro susceptibility testing, although transient expression of antimicrobial resistance by intracellular bacteria could still have occurred (1). Three other groups have reported on the clearance of L. pneumophila from the lung, using the same guinea pig model but with different bacterial inocula or routes of infection (16-18). Fitzgeorge and colleagues showed that azithromycin treatment (15 mg/kg i.p. or orally for 2 days) rapidly and completely cleared L. pneumophila from the lung for up to 3 days after the cessation of therapy, whereas no treatment resulted in bacterial concentrations of around log10 9 CFU/lung (16, 17). In the same study, treatment with erythromycin or clarithromycin (both given for 2 days) resulted in lung bacterial concentrations of about log10 6 to log10 7 CFU/lung 1 day after cessation of therapy and log10 2 to log10 6 CFU/lung 5 days after the end of therapy. García-de-Lomas and colleagues showed that erythromycin therapy given for 7 days resulted in log10 1 to log10 4 CFU/ml of lung at the end of therapy, whereas untreated animal lungs contained about log10 8 CFU/ml 4 days postinfection (18). Thus, except for very active antimicrobial agents, residual lung bacteria are a common finding in this animal model.
Interpretation of the results of the animal study on a pharmacodynamic basis is complicated by several factors. These include uncertainty about the “true” tigecycline MIC for F889; unknown tigecycline intracellular concentrations and subcellular location; and, most important, the unknown predictive value of MIC/AUC ratios for this animal model.
It is difficult to predict from these studies what the efficacy of tigecycline will be for the treatment of human Legionnaires' disease because of the uncertainties discussed above. Data from the guinea pig pneumonia model may not mimic the response of humans to antimicrobial therapy for Legionnaires' disease because of differences between the guinea pig and humans in drug pharmacodynamics, drug toxicities, severity of disease and immunocompromise, and other unknown factors. In general the animal model has been accurate at predicting which drugs will have no activity, good but not excellent activity, and superior activity in humans with Legionnaires' disease (2, 3; M. L. Pedro-Botet, Z. Vilaseca, N. Sopena, B. Viñado, E. Reynaga, M. García-Nuñez, and M. Sabria, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother, abstr. L-878, 2001). It is unclear whether the animal model can help to determine whether drugs within one of these three categories can be accurately ranked against one another in terms of their relative activities. Tigecycline falls into the intermediate (good but not excellent) category because it effectively prevents animal deaths from otherwise-fatal L. pneumophila pneumonia, because it effects bacterial clearance while the drug is being administered, and because of the supportive findings of intracellular activity similar to that of erythromycin. Based on these findings, tigecycline should be effective for the treatment of Legionnaires' disease of mild severity, but prolonged therapy (14 to 21 days) would be required for a cure, assuming that tigecycline dosing in humans achieves serum pharmacokinetic values comparable to those achieved in the guinea pig treatment study. However, tigecycline does not appear to be the drug of choice to treat severe Legionnaires' disease, such as that requiring hospitalization because of pneumonia severity, or for treatment of the disease in an immunocompromised patient. The same can be said of other tetracyclines, erythromycin, and clarithromycin.
Acknowledgments
This study was funded by Wyeth Research.
Baofeng Hu and Edward Schenck provided expert technical assistance.
REFERENCES
- 1.Barker, J., H. Scaife, and M. R. Brown. 1995. Intraphagocytic growth induces an antibiotic-resistant phenotype of Legionella pneumophila. Antimicrob. Agents Chemother. 39:2684-2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edelstein, P. H. 1995. Antimicrobial chemotherapy for legionnaires' disease: a review. Clin. Infect. Dis. 21:S265-S276. [DOI] [PubMed] [Google Scholar]
- 3.Edelstein, P. H. 1999. The guinea-pig model of Legionnaires' disease, p. 303-314. In O. Zak and M. A. Sande. (ed.), Handbook of animal models of infection. Academic Press, London, United Kingdom.
- 4.Edelstein, P. H., K. Calarco, and V. K. Yasui. 1984. Antimicrobial therapy of experimentally induced Legionnaires' disease in guinea pigs. Am. Rev. Respir. Dis. 130:849-856. [DOI] [PubMed] [Google Scholar]
- 5.Edelstein, P. H., and M. A. Edelstein. 1999. In vitro activity of the ketolide HMR 3647 (RU 6647) for Legionella spp., its pharmacokinetics in guinea pigs, and use of the drug to treat guinea pigs with Legionella pneumophila pneumonia. Antimicrob. Agents Chemother. 43:90-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edelstein, P. H., and M. A. Edelstein. 2000. In vitro activity of quinupristin/dalfopristin (Synercid, RP 59500) against Legionella spp. Diagn. Microbiol. Infect. Dis. 36:49-52. [DOI] [PubMed] [Google Scholar]
- 7.Edelstein, P. H., and M. A. C. Edelstein. 1989. WIN 57273 is bactericidal for Legionella pneumophila grown in alveolar macrophages. Antimicrob. Agents Chemother. 33:2132-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edelstein, P. H., and M. A. C. Edelstein. 1991. In vitro activity of azithromycin against clinical isolates of Legionella species. Antimicrob. Agents Chemother. 35:180-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelstein, P. H., and M. A. C. Edelstein. 1993. Comparison of three buffers used in the formulation of buffered charcoal yeast extract medium. J. Clin. Microbiol. 31:3329-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edelstein, P. H., M. A. C. Edelstein, K. H. Lehr, and J. Ren. 1996. In-vitro activity of levofloxacin against clinical isolates of Legionella spp., its pharmacokinetics in guinea pigs, and use in experimental Legionella pneumophila pneumonia. J. Antimicrob. Chemother. 37:117-126. [DOI] [PubMed] [Google Scholar]
- 11.Edelstein, P. H., M. A. C. Edelstein, J. J. Ren, R. Polzer, and R. P. Gladue. 1996. Activity of trovafloxacin (cp-99,219) against Legionella isolates: in vitro activity, intracellular accumulation and killing in macrophages, and pharmacokinetics and treatment of guinea pigs with L. pneumophila pneumonia. Antimicrob. Agents Chemother. 40:314-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edelstein, P. H., M. A. C. Edelstein, J. Weidenfeld, and M. B. Dorr. 1990. In vitro activity of sparfloxacin (CI-978; AT-4140) for clinical Legionella isolates, pharmacokinetics in guinea pigs, and use to treat guinea pigs with L. pneumophila pneumonia. Antimicrob. Agents Chemother. 34:2122-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edelstein, P. H., F. Higa, and M. A. C. Edelstein. 2001. In vitro activity of ABT-773 against Legionella pneumophila, its pharmacokinetics in guinea pigs, and its use to treat guinea pigs with L. pneumophila pneumonia. Antimicrob. Agents Chemother. 45:2685-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edelstein, P. H., T. Shinzato, E. Doyle, and M. A. Edelstein. 2001. In vitro activity of gemifloxacin (SB-265805, LB20304a) against Legionella pneumophila and its pharmacokinetics in guinea pigs with L. pneumophila pneumonia. Antimicrob. Agents Chemother. 45:2204-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edelstein, P. H., T. Shinzato, and M. A. Edelstein. 2001. BMS-284756 (T-3811ME) a new fluoroquinolone: in vitro activity against Legionella, efficacy in a guinea pig model of L. pneumophila pneumonia and pharmacokinetics in guinea pigs. J. Antimicrob. Chemother. 48:667-675. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgeorge, R. B., A. S. Featherstone, and A. Baskerville. 1990. Efficacy of azithromycin in the treatment of guinea pigs infected with Legionella pneumophila by aerosol. J. Antimicrob. Chemother. 25(Suppl. A):101-108. [DOI] [PubMed] [Google Scholar]
- 17.Fitzgeorge, R. B., S. Lever, and A. Baskerville. 1993. A comparison of the efficacy of azithromycin and clarithromycin in oral therapy of experimental airborne Legionnaires' disease. J. Antimicrob. Chemother. 31(Suppl. E):171-176. [DOI] [PubMed] [Google Scholar]
- 18.García-de-Lomas, J., E. Millas, M. A. Lazaro, M. Bermejo, C. Gimeno, D. Navarro, and S. Sanchez. 1998. A comparative study on the efficacy of the new quinolone alatrofloxacin in the treatment of experimental legionellosis in guinea pigs. Eur. J. Clin. Microbiol. Infect. Dis. 17:420-423. [DOI] [PubMed] [Google Scholar]
- 19.Stamler, D. A., M. A. C. Edelstein, and P. H. Edelstein. 1994. Azithromycin pharmacokinetics and intracellular concentrations in Legionella pneumophila-infected and uninfected guinea pigs and their alveolar macrophages. Antimicrob. Agents Chemother. 38:217-222. [DOI] [PMC free article] [PubMed] [Google Scholar]