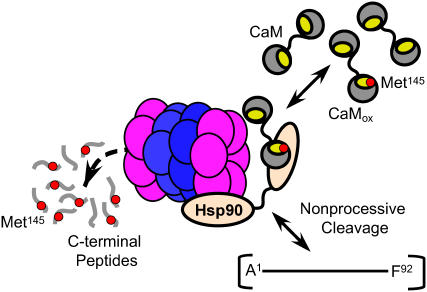
FIGURE 10.
Model highlighting role of Met145 as a sensor in mediating recognition and degradation of CaMox by proteasome. The 20S proteasome complex composed of rings of α- (purple) and β- (blue) subunits in association with Hsp90 oligomers (tan) preferentially recognizes and degrades CaMox after oxidation of Met145 to its sulfoxide (red dot). CaMox containing Met145 in hydrophobic binding pocket (yellow) is targeted for degradation; intermediates indicative of the nonprocessive cleavage of CaMox include large fragments A1-F89 (not shown) and A1-F92 as well as C-terminus peptides (G132–K148) enriched in Met(O)145. Similar intermediates involving large CaM fragments are observed upon site-directed substitution of Met144 and Met145 with leucines (Fig. 6), consistent with a structural sensitivity at positions 144 or 145.