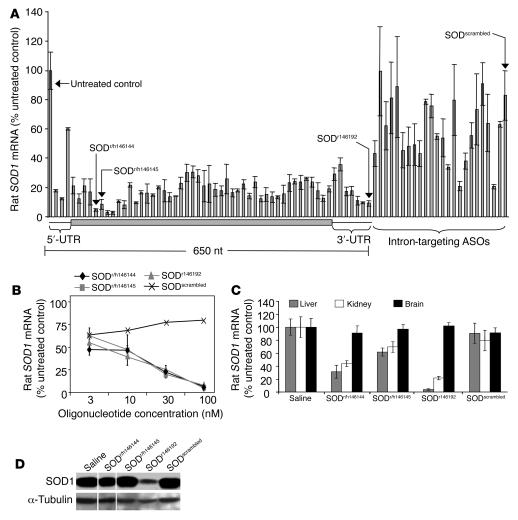
Figure 2. Identifying antisense oligonucleotides that reduce rat SOD1 in vitro and in vivo.
(A) We synthesized seventy-eight 24-mer, modified oligonucleotides complementary to rat SOD1 mRNA and transfected them at 150 nM into primary rat A10 cells. RNA was prepared 24 hours after transfection, and SOD1 mRNA levels were measured by quantitative RT-PCR. Oligonucleotides are displayed relative to their positions on the 462-nucleotide SOD1 coding sequence. Mean ± SD are shown (n = 4). UTR, untranslated region; ASOs, antisense oligonucleotides. (B) Oligonucleotides identified by the in vitro screen in A were evaluated in a similar transfection paradigm again using rat A10 cells and transfection of increasing concentrations of oligonucleotide to produce a dose-response curve. (C) Oligonucleotides SODr/h146144, SODr/h146145, SODr146192, and SODscrambled (a control oligonucleotide) were injected (37.5 mg/kg) 3 times per week intraperitoneally into adult rats for 3 weeks, after which time mRNA levels were measured in the liver, kidney, and brain. Mean ± SD are shown (n = 6). (D) SOD1 protein levels in liver extracts from animals treated with oligonucleotides SODr/h146144, SODr/h146145, SODr146192, and SODscrambled were measured by immunoblotting with an antibody to SOD1 (18). An immunoblot for tubulin was performed to verify protein loading.