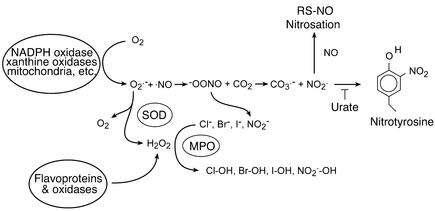
Figure 2.
Common reactions of oxygen radicals and nitric oxide in a biological setting. NADPH oxidase and xanthine oxidase transfer a single electron to oxygen to form superoxide anion, while other flavoproteins can transfer two electrons to form hydrogen peroxide directly. SODs scavenge superoxide, which forms additional hydrogen peroxide. Peroxidases use hydrogen peroxide to oxidize a wide range of substrates to produce many different reactive species. Nitric oxide reacts with superoxide to form peroxynitrite. Carbon dioxide reacts catalytically with peroxynitrite to form nitrogen dioxide and carbonate radical. These two radicals nitrate tyrosine, which is blocked by the competitive inhibitor urate. Nitric oxide can also react with these radicals to form nitrosating intermediates that can produce, among other things, S-nitrosothiols. MPO, myeloperoxidase; RS-NO, S-nitrosothiols.