Abstract
The human brain forms over an unusually long period compared to other organs. While most of the basic structure is laid down before birth, neuron proliferation and migration continue in the postnatal period. The blood-brain barrier is not fully developed until the middle of the first year of life. The number of synaptic connections between neurons reaches a peak around age two and is then trimmed back by about half. Similarly, there is great postnatal activity in the development of receptors and transmitter systems as well as in the production of myelin. Many of the toxic agents known to damage the developing brain interfere with one or more of these developmental processes. Those with antimitotic action, such as X-ray and methyl mercury, have distinctly different effects on structure depending on which neurons are forming at the time of exposure. Vulnerability to agents that interfere with cell production decreases rapidly over the early postnatal period. Other toxic substances, such as psychoactive drugs and agents that alter hormone levels, are especially hazardous during synaptogenesis and the development of transmitter systems, and thus continue to be damaging for years after birth. Still other toxic substances such as lead, seem to have their greatest effects during even later stages of brain development, perhaps by interfering with the trimming back of connections. Guidelines designed to protect human populations from developmental neurotoxicity need to take into account the changing sensitivity of the brain as it passes through different developmental stages, as well as the fundamental differences in the effects of toxicants on the mature and the developing brain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adinolfi M. The development of the human blood-CSF-brain barrier. Dev Med Child Neurol. 1985 Aug;27(4):532–537. doi: 10.1111/j.1469-8749.1985.tb04581.x. [DOI] [PubMed] [Google Scholar]
- Amin-Zaki L., Elhassani S., Majeed M. A., Clarkson T. W., Doherty R. A., Greenwood M. Intra-uterine methylmercury poisoning in Iraq. Pediatrics. 1974 Nov;54(5):587–595. [PubMed] [Google Scholar]
- Barks J. D., Silverstein F. S., Sims K., Greenamyre J. T., Johnston M. V. Glutamate recognition sites in human fetal brain. Neurosci Lett. 1988 Jan 22;84(2):131–136. doi: 10.1016/0304-3940(88)90396-5. [DOI] [PubMed] [Google Scholar]
- Bauman M., Kemper T. L. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985 Jun;35(6):866–874. doi: 10.1212/wnl.35.6.866. [DOI] [PubMed] [Google Scholar]
- Bedi K. S., Thomas Y. M., Davies C. A., Dobbing J. Synapse-to-neuron ratios of the frontal and cerebellar cortex of 30-day-old and adult rats undernourished during early postnatal life. J Comp Neurol. 1980 Sep 1;193(1):49–56. doi: 10.1002/cne.901930104. [DOI] [PubMed] [Google Scholar]
- Black I. B. Stages of neurotransmitter development in autonomic neurons. Science. 1982 Mar 5;215(4537):1198–1204. doi: 10.1126/science.215.4537.1198. [DOI] [PubMed] [Google Scholar]
- Burbacher T. M., Rodier P. M., Weiss B. Methylmercury developmental neurotoxicity: a comparison of effects in humans and animals. Neurotoxicol Teratol. 1990 May-Jun;12(3):191–202. doi: 10.1016/0892-0362(90)90091-p. [DOI] [PubMed] [Google Scholar]
- Choi B. H., Lapham L. W., Amin-Zaki L., Saleem T. Abnormal neuronal migration, deranged cerebral cortical organization, and diffuse white matter astrocytosis of human fetal brain: a major effect of methylmercury poisoning in utero. J Neuropathol Exp Neurol. 1978 Nov-Dec;37(6):719–733. doi: 10.1097/00005072-197811000-00001. [DOI] [PubMed] [Google Scholar]
- Cooper L. Z., Krugman S. Clinical manifestations of postnatal and congenital rubella. Arch Ophthalmol. 1967 Apr;77(4):434–439. doi: 10.1001/archopht.1967.00980020436004. [DOI] [PubMed] [Google Scholar]
- Cragg B. G. The development of synapses in the visual system of the cat. J Comp Neurol. 1975 Mar 15;160(2):147–166. doi: 10.1002/cne.901600202. [DOI] [PubMed] [Google Scholar]
- EAYRS J. T. The cerebral cortex of normal and hypothyroid rats. Acta Anat (Basel) 1955;25(2-4):160–183. doi: 10.1159/000141068. [DOI] [PubMed] [Google Scholar]
- Gray L. E., Jr, Kavlock R. J., Chernoff N., Ferrell J., McLamb J., Ostby J. Prenatal exposure to the herbicide 2,4-dichlorophenyl-p-nitrophenyl ether destroys the rodent Harderian gland. Science. 1982 Jan 15;215(4530):293–294. doi: 10.1126/science.7053576. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P. R., de Courten C. The development of synapses in striate cortex of man. Hum Neurobiol. 1987;6(1):1–9. [PubMed] [Google Scholar]
- Jones K. L., Smith D. W., Ulleland C. N., Streissguth P. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973 Jun 9;1(7815):1267–1271. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- Lauder J. M. Neurotransmitters as morphogens. Prog Brain Res. 1988;73:365–387. doi: 10.1016/S0079-6123(08)60516-6. [DOI] [PubMed] [Google Scholar]
- Levin A. A., Miller R. K. Dept. of Pharmacology & Toxicology, University of Rochester, School of Medicine and Dentistry, New York. Teratology. 1980 Aug;22(1):1–5. doi: 10.1002/tera.1420220102. [DOI] [PubMed] [Google Scholar]
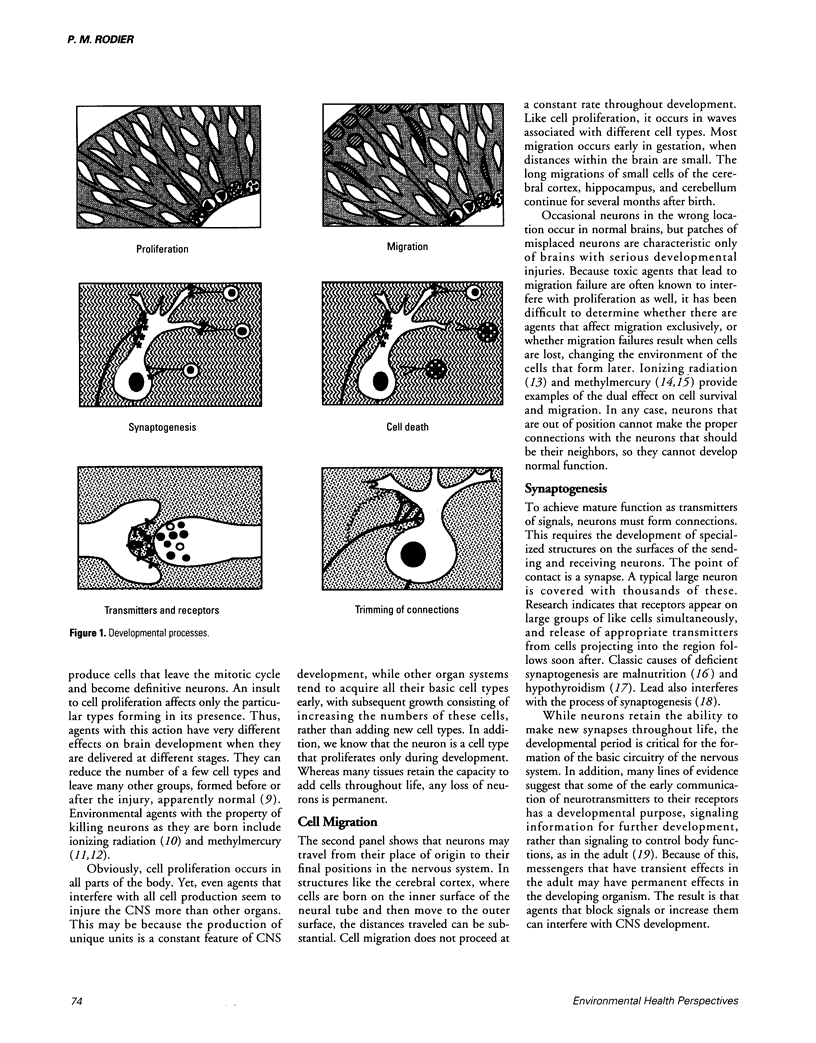
- McDonald J. W., Johnston M. V. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res Brain Res Rev. 1990 Jan-Apr;15(1):41–70. doi: 10.1016/0165-0173(90)90011-c. [DOI] [PubMed] [Google Scholar]
- Miller M. T. Thalidomide embryopathy: a model for the study of congenital incomitant horizontal strabismus. Trans Am Ophthalmol Soc. 1991;89:623–674. [PMC free article] [PubMed] [Google Scholar]
- Needleman H. L., Schell A., Bellinger D., Leviton A., Allred E. N. The long-term effects of exposure to low doses of lead in childhood. An 11-year follow-up report. N Engl J Med. 1990 Jan 11;322(2):83–88. doi: 10.1056/NEJM199001113220203. [DOI] [PubMed] [Google Scholar]
- Price M. T., Olney J. W., Lowry O. H., Buchsbaum S. Uptake of exogenous glutamate and aspartate by circumventricular organs but not other regions of brain. J Neurochem. 1981 May;36(5):1774–1780. doi: 10.1111/j.1471-4159.1981.tb00430.x. [DOI] [PubMed] [Google Scholar]
- Rodier P. M., Aschner M., Sager P. R. Mitotic arrest in the developing CNS after prenatal exposure to methylmercury. Neurobehav Toxicol Teratol. 1984 Sep-Oct;6(5):379–385. [PubMed] [Google Scholar]
- Rodier P. M. Chronology of neuron development: animal studies and their clinical implications. Dev Med Child Neurol. 1980 Aug;22(4):525–545. doi: 10.1111/j.1469-8749.1980.tb04363.x. [DOI] [PubMed] [Google Scholar]
- Rosa F. W. Teratogenicity of isotretinoin. Lancet. 1983 Aug 27;2(8348):513–513. doi: 10.1016/s0140-6736(83)90538-x. [DOI] [PubMed] [Google Scholar]
- Rosengarten H., Friedhoff A. J. Enduring changes in dopamine receptor cells of pups from drug administration to pregnant and nursing rats. Science. 1979 Mar 16;203(4385):1133–1135. doi: 10.1126/science.570724. [DOI] [PubMed] [Google Scholar]
- Sager P. R., Doherty R. A., Rodier P. M. Effects of methylmercury on developing mouse cerebellar cortex. Exp Neurol. 1982 Jul;77(1):179–193. doi: 10.1016/0014-4886(82)90152-2. [DOI] [PubMed] [Google Scholar]
- Schull W. J., Norton S., Jensh R. P. Ionizing radiation and the developing brain. Neurotoxicol Teratol. 1990 May-Jun;12(3):249–260. doi: 10.1016/0892-0362(90)90096-u. [DOI] [PubMed] [Google Scholar]
- Wiggins R. C. Myelin development and nutritional insufficiency. Brain Res. 1982 Jun;257(2):151–175. doi: 10.1016/0165-0173(82)90016-9. [DOI] [PubMed] [Google Scholar]


