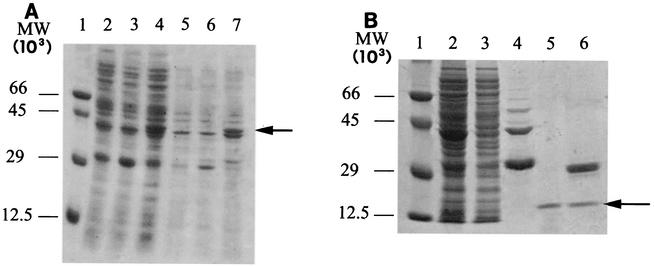
FIG. 4.
SDS-PAGE analysis of GST-Lys protein expression (A) and purification (B). (A) E. coli BL21 cells containing the pGEX-lys plasmid were grown at 30°C to an optical density at 600 nm of 1.0 and then induced with 0.1 mM IPTG for 4 h. Lane 1 contained molecular weight (MW) standards; the values on the left indicate molecular weights. Lane 2, crude soluble extract from nontransformed BL21 host cells; lane 3, crude soluble extract from BL21 cells containing nonrecombinant plasmid pGEX; lane 4, crude soluble extract from BL21 host cells containing the pGEX-lys recombinant plasmid; lane 5, solubilized pellet from nontransformed BL21 cells; lane 6, solubilized pellet from BL21 cells transformed with the nonrecombinant pGEX plasmid; lane 7, solubilized inclusion bodies from BL21 cells containing the pGEX-lys recombinant plasmid. (B) Recombinant protein was purified by affinity chromatography on glutathione-Sepharose 4B resin and digested with 0.5 U of thrombin per μg of fusion protein. Lane 1 contained molecular weight markers. Lane 2, 20 μg of total proteins from BL21(pGEX-lys) cells sonicated before affinity purification; lane 3, 20 μg of total proteins from sonicated BL21(pGEX-lys) cells, after loading on affinity resin; lane 4, eluate from glutathione-Sepharose 4B resin; lane 5, flowthrough following thrombin digestion of GST-Lys fusion protein bound to glutathione-Sepharose 4B; lane 6, thrombin digest of eluate from glutathione-Sepharose 4B resin. The arrow indicates the position of the 13.5-kDa recombinant Lys protein.