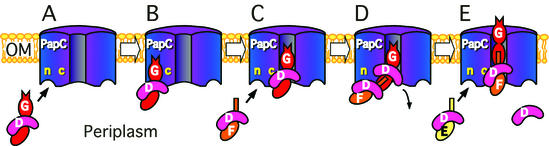
FIG. 8.
Model for P pilus biogenesis by the PapC usher. The oligomeric usher complex contains N-terminal sites (n) and C-terminal sites (c) for interaction with chaperone-subunit complexes. (A and B) Periplasmic chaperone-subunit complexes target the N-terminal usher site. The PapDG chaperone-adhesin complex has the highest affinity for the usher and targets first. (C) PapDG moves from the N-terminal targeting site to the C-terminal assembly site, forming a stable assembly intermediate. The N-terminal site is now open for targeting of the next chaperone-subunit complex, PapDF, to the usher. (D) The N-terminal extension of the bound PapF subunit undergoes donor strand exchange with the subunit domain of PapG, causing uncapping of the chaperone from PapG. (E) Donor strand exchange between PapF and PapG generates the first link in the pilus tip. This results in a shift of the PapDF complex to the usher C-terminal assembly site, allowing targeting of the next chaperone-subunit complex, PapDE, to the usher. Repeated cycles of targeting and assembly would result in extension and secretion of the pilus tip and rod.