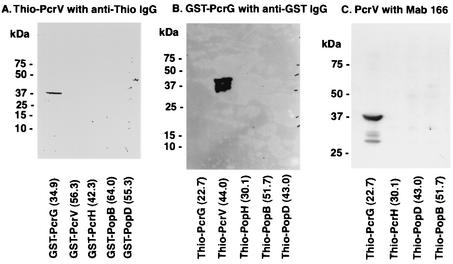
FIG. 1.
Affinity immunoblot analysis. (A) Binding of Thio-PcrV to GST-PcrG. The protein samples from induced E. coli clones carrying pGEX plasmids were electrophoresed onto a sodium dodecyl sulfate-4 to 12% bis-Tris polyacrylamide gel, electroblotted onto a nitrocellulose membrane, and incubated with E. coli lysate including expressed Thio-PcrV. The membrane was developed with anti-Thio immunoglobulin G (IgG) and secondary anti-mouse IgG conjugated with horseradish peroxidase and a chemiluminescent substrate. An intense isolated band represents binding of GST-PcrG to Thio-PcrV. (B) Binding of GST-PcrG to Thio-PcrV. The protein samples were from induced E. coli clones carrying pThio plasmids. The blotted membrane was incubated with recombinant GST-PcrG (10 μg/ml) and then developed with anti-GST IgG conjugated with horseradish peroxidase and a chemiluminescent substrate. An intense isolated band represents binding of GST-PcrG to Thio-PcrV. (C) Binding of PcrV to Thio-PcrG. The protein samples were from induced E. coli clones carrying pThio plasmids. The membrane was incubated with recombinant PcrV (10 μg/ml), then developed with murine anti-PcrV MAb 166, and developed with secondary anti-mouse antibodies conjugated with horseradish peroxidase and a chemiluminescent substrate. An intense isolated band represents binding of PcrV to Thio-PcrG.