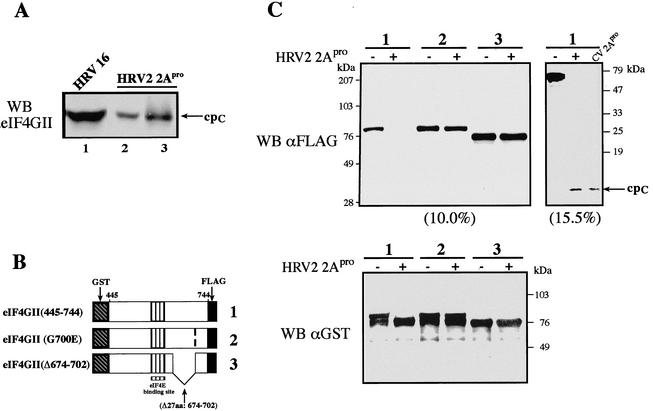
FIG. 1.
(A) Identification of the 2Apro cleavage site in eIF4GII. HeLa-I cells were infected with HRV16 (100 50% tissue culture infective doses per cell) as described previously (30). Cell extract (60 μg of protein; lane 1) was prepared 6 h postinfection and loaded on a gel in parallel with 40 μg of HeLa S10 (lane 2) or purified recombinant eIF4GII (1/40 of the reaction mixture, ∼0.5 μg; lane 3) that was incubated in the presence of purified HRV2 2Apro. Proteins were resolved by SDS-8% PAGE and blotted onto nitrocellulose. The blot was treated with polyclonal antibodies against the cpC of eIF4GII. WB, Western blot. (B and C) eIF4GII mutants that are resistant to HRV2 2Apro cleavage. (B) Scheme of wild-type eIF4GII (1) and mutant (2 and 3) fragments. GST-eIF4GII (aa 445 to 744)-FLAG fragments contained a single point mutation (G700E) or a deletion (Δ674-702). (C) eIF4GII proteins were expressed in E. coli and purified on a glutathione-Sepharose column (4). Purified proteins (0.5 μg) were incubated in the presence or absence of recombinant 2Apro (2.8 μg) in buffer containing 100 mM potassium acetate, 20 mM Tris-HCl (pH 7.6), 2.5 mM magnesium acetate, and 10% glycerol for 1 h at 30°C. Laemmli buffer was added to stop the reaction. Samples were resolved by SDS-PAGE (10.0, 15.5, or 12.5% [bottom] acrylamide). Proteins were transferred onto a nitrocellulose membrane, which was subsequently incubated with anti-GST or anti-FLAG antibodies as indicated. The small (62-aa) C-terminal cleavage product containing the FLAG epitope was resolved only in a 15.5% gel (cpC). The wild-type eIF4GII fragment was cleaved by HRV2 or CVB4 2Apro, as indicated. Positions of molecular mass standards are shown on the right.