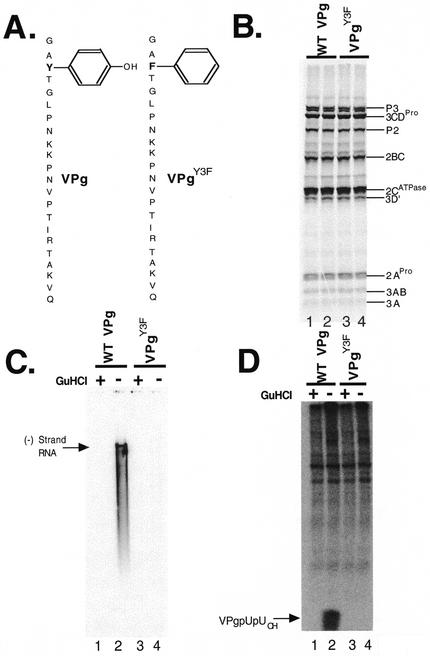
FIG. 4.
VPg is required for negative-strand RNA synthesis. Poliovirus protein synthesis, negative-strand RNA synthesis, and VPg uridylylation were assayed as described in Materials and Methods. Poliovirus RNA2 replicons with two 5′-terminal nonviral guanosines encoding wild-type (WT) or Y3F mutant VPg (VPgY3F) were used. (A) Diagrams of wild-type VPg and VPgY3F. (B) SDS-PAGE. Poliovirus proteins were synthesized in reaction mixtures containing [35S]methionine and the wild-type (WT) RNA2 replicon (duplicate samples in lanes 1 and 2) or VPgY3F replicon (duplicate samples in lanes 3 and 4). Radiolabeled proteins were fractionated by SDS-PAGE and detected by phosphorimaging. (C) Negative-strand RNA synthesis. Poliovirus RNA was synthesized in reaction mixtures containing [α-32P]CTP by preinitiation RNA replication complexes containing wild-type poliovirus RNA2 templates (lanes 1 and 2) or VPgY3F RNA templates (lanes 3 and 4). Reactions were performed in the presence (lanes 1 and 3) and absence (lanes 2 and 4) of 2 mM guanidine HCl. Products from the reactions were fractionated by electrophoresis in a denaturing methylmercury hydroxide agarose gel. Radiolabled negative-strand RNA in the gel was detected by phosphorimaging. (D) VPg uridylylation. VPgpUpUOH was synthesized in reaction mixtures containing [α-32P]UTP by preinitiation RNA replication complexes containing wild-type poliovirus RNA templates (lanes 1 and 2) or mutant VPgY3F RNA templates (lanes 3 and 4). Reactions were performed in the presence (lanes 1 and 3) and absence (lanes 2 and 4) of 2 mM guanidine HCl. Products from the reactions were fractionated by polyacrylamide-Tris-Tricine gel electrophoresis and detected by phosphorimaging.