FIG. 4.
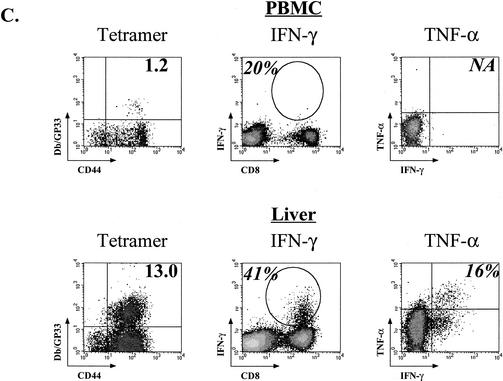
Progressive loss of cytokine production during chronic LCMV Cl-13 infection. (A) The fraction of tetramer-positive cells able to produce IFN-γ (top row) was determined for the NP396-, GP33-, and GP276-specific populations at the indicated times p.i. with LCMV Cl-13 (Chronic) or Armstrong (Acute). The percentage of the tetramer-positive population able to produce TNF-α (middle row) or IL-2 (bottom row) was determined using three-color intracellular cytokine staining. Asterisks indicate too few cells available for analysis as a result of deletion. Open bars indicate LCMV Armstrong infection, while the black bars indicate LCMV Cl-13 infection. The data represent the average and standard deviation for three to six mice/time point. (B) An example of fully functional GP33-specific memory CD8 T cells from an LCMV Armstrong-immune mouse (top row) and three examples of different states of functional exhaustion of GP33-specific T cells during LCMV Cl-13 infection are shown. The percentage in the left column indicates the percentage of CD8 T cells that is Db/GP33 tetramer-positive. The percentage in the second, third, or fourth column indicates the percentage of tetramer-positive cells that is IFN-γ+, TNF-α+, or IL-2+, respectively. Plots for tetramer and IFN-γ staining are gated on all lymphocytes. Plots for TNF-α and IL-2 are gated on CD8 T cells. Similar results were observed for the GP276 response (data not shown). (C) Lymphocytes from the PBMC (upper panels) or intrahepatic lymphocytes (lower panels) of LCMV Cl-13-infected mice (chronic; day 35 p.i. for PBMC, day 60 p.i. for liver) were examined for functional exhaustion. The left columns show the percent Db/GP33 tetramer-positive cells (numbers indicate percentages of CD8 T cells). Production of IFN-γ (gated on all lymphocytes) and TNF-α (gated on CD8 T cells) is shown in the middle and right columns, respectively. Numbers indicate the percentage of tetramer-positive cells making cytokine. No IL-2-producing cells were detected from CD8 T cells from chronically infected mice (data not shown). Similar results were observed at other time points and for GP276-specific responses (data not shown). Note that the GP33 peptide used for intracellular cytokine staining stimulates both the Db/GP33 and Kb/GP34 response. Therefore, the percent functional of tetramer-positive cells shown in panels A, B, and C was determined using both DbGP33 and KbGP34 tetramers. As shown in Fig. 2, the Kb/GP34 becomes negligible by 2 to 3 weeks p.i. Therefore, only the Db/GP33 tetramer stain is shown. No specific staining was observed when using isotype control antibodies.