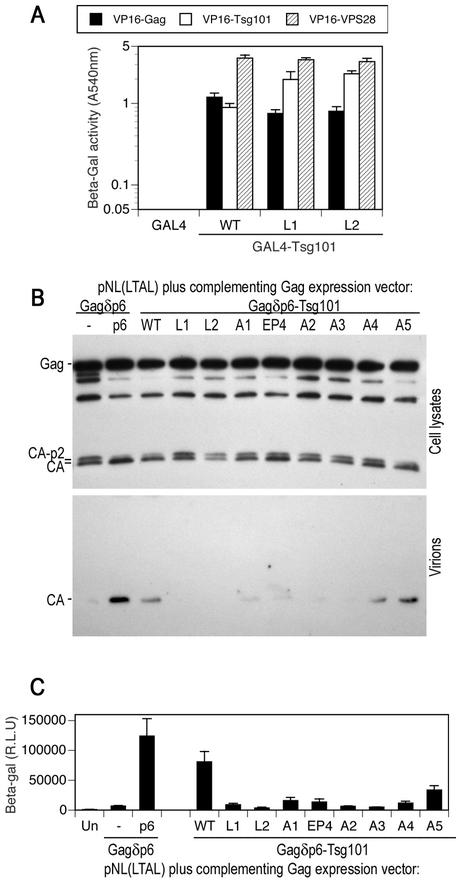
FIG. 5.
Leucine zipper, multimerization, and VPS28 binding domains of Tsg101 are required to mediate HIV-1 virion budding. (A) Y190 yeast cells were transformed with plasmids expressing wild-type (WT) or leucine zipper mutant GAL4-Tsg101 proteins as well as VP16-HIV-1 Gag, VP16-Tsg101, or VP16-VPS28, as indicated. The mean level of β-galactosidase (β-Gal) expression (± standard deviation) in three pools of transformants is shown. (B) HOS cells were transfected with an L-domain-defective HIV-1 proviral plasmid, pNL(LTAL), and a complementing expression vector. The complementing vector expressed a Gagδp6 protein alone (lane −) or a Gagδp6 protein to which either p6 or wild-type or mutant forms of Tsg101 were fused at the C terminus. Cell lysates and extracellular particles were analyzed by Western blotting with an anti-HIV-1 CA antibody. (C) Culture supernatants from HOS cells transfected as for panel B were analyzed for the presence of infectious virions by inoculation of P4/R5 indicator cells. β-Galactosidase activity in cell lysates was measured 48 h after inoculation, and the mean and standard deviation are shown. The background β-galactosidase activity in lysates of uninfected P4/R5 cells (Un) was approximately 1,000 relative light units (R.L.U.).