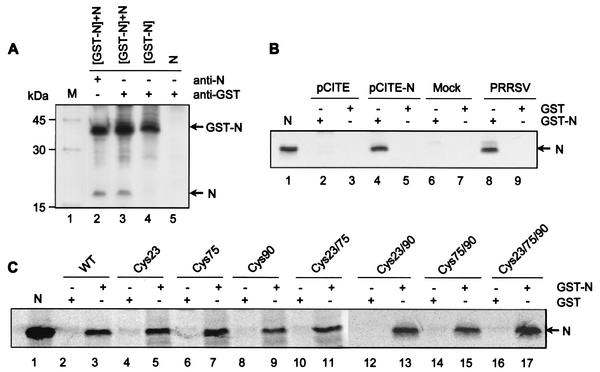
FIG. 7.
Homotypic interactions between N proteins in vitro. (A) Coimmunoprecipitation of the N protein. HeLa cells infected with vTF7-3 were cotransfected with plasmids expressing the recombinant N protein alone (N) or as a GST fusion protein (GST-N). The cells were radiolabeled with [35S]methionine and lysed using a nonionic detergent (see Materials and Methods). The cell lysates were immunoprecipitated under reducing conditions in the presence (+) of nonionic detergents with either a mixture of N-specific MAbs (lane 2) or an anti-GST polyclonal antibody (lanes 3, 4, and 5) and analyzed by SDS-PAGE. Lanes 2 and 3, cotransfection of both GST-N and N gene constructs; lane 4, transfection of GST-N gene construct; lane 5, transfection of N gene construct. (B) GST pull-down assay. Bacterially expressed GST-N (lanes 2, 4, 6, and 8) or GST (lanes 3, 5, 7, and 9) were bound to glutathione-Sepharose beads and incubated with either in vitro-translated N protein labeled with [35S]methionine (lanes 4 and 5) or virus-infected cell lysates metabolically labeled with [35S]methionine (lanes 8 and 9). The beads were washed four times, and the bound proteins were eluted by boiling them in reducing sample buffer, followed by SDS-PAGE and autoradiography. The N protein immunoprecipitated from virus-infected cells was run in lane 1 as a marker. In vitro translation products from empty plasmid (lanes 2 and 3) and from mock-infected cell lysates (lanes 6 and 7) were incubated with the bead-bound proteins as a control. (C) GST pull-down assay examining the interaction between N protein cysteine mutants and the wild-type (WT) N protein in vitro. Bacterially expressed GST and GST-N proteins coupled to glutathione-Sepharose beads and incubated with individual cysteine mutants of the N protein synthesized by in vitro translation are shown. N protein immunoprecipitated from PRRSV-infected cells was run as a marker (lane 1).