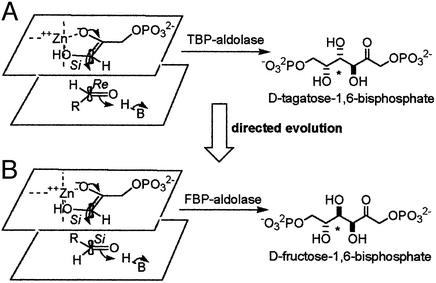
Figure 2.
Stereochemistry of the reaction catalyzed by aldolases. (A) The mechanism of TBP aldolase. The DHAP ene-diolate is formed after abstraction of the 1-proS proton from DHAP and polarization by the catalytic zinc cation. Attack of the activated DHAP C1 from its Si face onto the G3P C1 Re face generates the 3S, 4S product tagatose 1,6-bisphosphate, and proton donation by H-B (Asp-82) (32) converts the C4 carbonyl to a hydroxyl group, completing TBP synthesis. (B) The mechanism of FBP aldolase. The DHAP ene-diolate is formed after abstraction of the 1-proS proton from DHAP (27) by Glu-182 (26) and polarization by the catalytic zinc. Attack of the activated DHAP C1 from its Si face onto the G3P C1 Si face and proton donation by H-B (Asp-109) (24) convert the C4 carbonyl to a hydroxyl group, completing the synthesis of the 3S, 4R product fructose 1,6-bisphosphate. FBP and TBP are epimeric at C4, and this position is marked with an asterisk. R = CH(OH)CH2OPO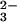 .
.