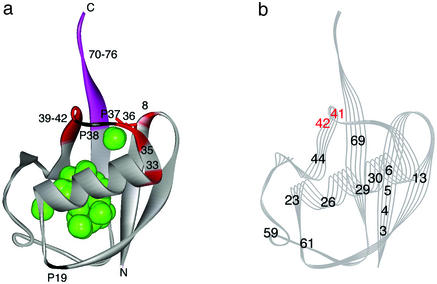
Figure 4.
(a) A qualitative presentation of the structure of the pressure-stabilized equilibrium intermediate I, based on 15N/1H HSQC crosspeak intensities of individual amide groups. The intensities of residues 8, 33, 35, 36, and 39–42 (red) are preferentially decreased above 2,000 bar at pH 4.5 at 0°C, showing local unfolding, whereas the rest of the molecule (gray) remains folded. The particularly labile residue Ile-36 is represented by a red stick. The C-terminal residues (70–76; purple-colored) are intrinsically disordered. The positions of all Pro residues (19, 37, and 38) are marked. Green spheres show cavities calculated with the program grasp (16) with a probe radius of 1.0 Å. (b) A qualitative presentation of the structure of the slow folding intermediate of ubiquitin, based on the results of a pulse-labeling 1H/2H exchange NMR experiment at pH 5.0 at 25°C by Briggs and Roder (9). The only residues for which 1H/2H exchange rates could be evaluated are numbered. The black-numbered residues are folded very quickly (within ≈10 ms), whereas the red-numbered residues (Gln-41 and Arg-42) remain unfolded for as long as ≈10 s, because of the slow transformation of a cis conformer of Pro-37 and/or Pro-38 peptide bond into trans (9). The figures are drawn with WebLab VIEWERLITE 3.2, based on the three-dimensional crystal structure of human ubiquitin (PDB ID code 1UBQ; ref. 2).